「デキストロアンフェタミン」の版間の差分
削除された内容 追加された内容
Wakkie1379 (会話 | 投稿記録) ページ「User:Mr. Ibrahem/Dextroamphetamine」の翻訳により作成 |
Wakkie1379 (会話 | 投稿記録) カテゴリー追加 |
||
| 1行目: | 1行目: | ||
{{Drugbox |
|||
| Verifiedfields = changed |
|||
| Watchedfields = changed |
|||
| verifiedrevid = 596899008 |
|||
| INN = |
|||
| image = D-amphetamine.svg |
|||
| width = 200px |
|||
<!--Names--> |
|||
{{Drugbox|image=D-amphetamine.svg|width=200px |
|||
| pronounce = {{IPAc-en|ˌ|d|ɛ|k|s|t|r|əʊ|æ|m|ˈ|f|ɛ|t|ə|m|iː|n}} |
|||
| tradename = Dexedrine, DextroStat, Metamina, Attentin, Zenzedi, others |
|||
| ⚫ | |||
| IUPAC_name = (2''S'')-1-Phenylpropan-2-amine |
|||
<!-- Clinical data --> |
|||
| ⚫ | |||
| class = [[Amphetamine]]<ref name=AHFS2021>{{cite web |title=Dextroamphetamine Monograph for Professionals |url=https://www.drugs.com/monograph/dextroamphetamine.html |website=Drugs.com |accessdate=28 December 2021 |language=en |archive-date=11 August 2021 |archive-url=https://web.archive.org/web/20210811223953/https://www.drugs.com/monograph/dextroamphetamine.html |url-status=live }}</ref> |
|||
| uses = [[attention deficit hyperactivity disorder|ADHD]], [[narcolepsy]]<ref name=BNF81>{{cite book |title=BNF 81: March-September 2021 |date=2021 |publisher=BMJ Group and the Pharmaceutical Press |isbn=978-0857114105 |page=370}}</ref> |
|||
| side_effects = Stomach pain, trouble sleeping, [[palpitations]], high blood pressure, anxiety, tremor, diarrhea<ref name=AHFS2021>{{cite web |title=Dextroamphetamine Monograph for Professionals |url=https://www.drugs.com/monograph/dextroamphetamine.html |website=Drugs.com |accessdate=28 December 2021 |language=en |archive-date=11 August 2021 |archive-url=https://web.archive.org/web/20210811223953/https://www.drugs.com/monograph/dextroamphetamine.html |url-status=live }}</ref> |
|||
| interactions = <!-- notable interactions --> |
|||
| pregnancy_AU = B3 |
|||
| pregnancy_US = C |
|||
| breastfeeding = |
|||
| routes_of_administration= [[Oral administration|By mouth]] |
|||
| onset = {{abbr|IR|Immediate release}} dosing: 0.5–1.5 hours<ref>{{Cite book|title = Primary Care Pediatrics|url = https://books.google.com/books?id=o43u_qWT4asC|publisher = Lippincott Williams & Wilkins|date = 1 January 2001|isbn = 9780781720083|first1 = Carol|last1 = Green-Hernandez|first2 = Joanne K.|last2 = Singleton|first3 = Daniel Z.|last3 = Aronzon|page = 243|access-date = 8 August 2021|archive-date = 23 May 2016|archive-url = https://web.archive.org/web/20160523193423/https://books.google.com/books?id=o43u_qWT4asC|url-status = live}}</ref> |
|||
| duration_of_action= {{abbr|IR|Immediate release}} dosing: 3–6 hours<ref name="Millichap: onset, peak, and duration"/><ref name="Narcolepsy guide">{{cite journal | vauthors = Mignot EJ | title = A practical guide to the therapy of narcolepsy and hypersomnia syndromes | journal = Neurotherapeutics | volume = 9 | issue = 4 | pages = 739–752 | date = October 2012 | pmid = 23065655 | pmc = 3480574 | doi = 10.1007/s13311-012-0150-9 }}</ref><br />{{abbr|XR|Extended release}} dosing: 8–12 hours<ref name="Stahl's Essential Psychopharmacology - dextroamphetamine">{{cite book | vauthors=Stahl SM | title=Prescriber's Guide: Stahl's Essential Psychopharmacology | date=March 2017 | publisher=Cambridge University Press | location=Cambridge, United Kingdom | isbn=9781108228749 | pages=39–44 | edition=6th | chapter-url=https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA39 | chapter=Amphetamine (D) | access-date=8 August 2017 }}</ref><ref name="Millichap: onset, peak, and duration"/><ref name="Narcolepsy guide">{{cite journal | vauthors = Mignot EJ | title = A practical guide to the therapy of narcolepsy and hypersomnia syndromes | journal = Neurotherapeutics | volume = 9 | issue = 4 | pages = 739–752 | date = October 2012 | pmid = 23065655 | pmc = 3480574 | doi = 10.1007/s13311-012-0150-9 }}</ref> |
|||
| defined_daily_dose= |
|||
| typical_dose = |
|||
| drug_name = |
|||
<!--External links--> |
|||
<!-- Clinical data -->|pregnancy_AU=B3|pregnancy_US=C|routes_of_administration=[[Oral administration|By mouth]]|onset={{abbr|IR|Immediate release}} dosing: 0.5–1.5 hours<ref>{{Cite book|title = Primary Care Pediatrics|url = https://books.google.com/books?id=o43u_qWT4asC|publisher = Lippincott Williams & Wilkins|date = 1 January 2001|isbn = 9780781720083|first1 = Carol|last1 = Green-Hernandez|first2 = Joanne K.|last2 = Singleton|first3 = Daniel Z.|last3 = Aronzon|page = 243|access-date = 8 August 2021|archive-date = 23 May 2016|archive-url = https://web.archive.org/web/20160523193423/https://books.google.com/books?id=o43u_qWT4asC|url-status = live}}</ref>|duration_of_action={{abbr|IR|Immediate release}} dosing: 3–6 hours<ref name="Millichap: onset, peak, and duration" /><ref name="Narcolepsy guide">{{cite journal | vauthors = Mignot EJ | title = A practical guide to the therapy of narcolepsy and hypersomnia syndromes | journal = Neurotherapeutics | volume = 9 | issue = 4 | pages = 739–752 | date = October 2012 | pmid = 23065655 | pmc = 3480574 | doi = 10.1007/s13311-012-0150-9 }}</ref><br />{{abbr|XR|Extended release}} dosing: 8–12 hours<ref name="Stahl's Essential Psychopharmacology - dextroamphetamine">{{cite book | vauthors=Stahl SM | title=Prescriber's Guide: Stahl's Essential Psychopharmacology | date=March 2017 | publisher=Cambridge University Press | location=Cambridge, United Kingdom | isbn=9781108228749 | pages=39–44 | edition=6th | chapter-url=https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA39 | chapter=Amphetamine (D) | access-date=8 August 2017 }}</ref><ref name="Millichap: onset, peak, and duration" /><ref name="Narcolepsy guide">{{cite journal | vauthors = Mignot EJ | title = A practical guide to the therapy of narcolepsy and hypersomnia syndromes | journal = Neurotherapeutics | volume = 9 | issue = 4 | pages = 739–752 | date = October 2012 | pmid = 23065655 | pmc = 3480574 | doi = 10.1007/s13311-012-0150-9 }}</ref>|drug_name=<!--External links-->|Drugs.com={{drugs.com|monograph|dextroamphetamine}}|MedlinePlus=a605027|dependency_liability=Moderate-high<ref name="Stahl's Essential Psychopharmacology">{{cite book | vauthors=Stahl SM | title=Prescriber's Guide: Stahl's Essential Psychopharmacology | date=March 2017 | publisher=Cambridge University Press | location=Cambridge, United Kingdom | isbn=9781108228749 | pages=45–51 | edition=6th | chapter-url=https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA45#v=onepage | chapter=Amphetamine (D,L) | access-date=5 August 2017 | archive-date=8 June 2019 | archive-url=https://web.archive.org/web/20190608044902/https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA45#v=onepage | url-status=live }}</ref><ref name="Stahl's Essential Psychopharmacology - dextroamphetamine">{{cite book | vauthors=Stahl SM | title=Prescriber's Guide: Stahl's Essential Psychopharmacology | date=March 2017 | publisher=Cambridge University Press | location=Cambridge, United Kingdom | isbn=9781108228749 | pages=39–44 | edition=6th | chapter-url=https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA39 | chapter=Amphetamine (D) | access-date=8 August 2017 }}</ref>|addiction_liability=Moderate-high<ref name="Stahl's Essential Psychopharmacology">{{cite book | vauthors=Stahl SM | title=Prescriber's Guide: Stahl's Essential Psychopharmacology | date=March 2017 | publisher=Cambridge University Press | location=Cambridge, United Kingdom | isbn=9781108228749 | pages=45–51 | edition=6th | chapter-url=https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA45#v=onepage | chapter=Amphetamine (D,L) | access-date=5 August 2017 | archive-date=8 June 2019 | archive-url=https://web.archive.org/web/20190608044902/https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA45#v=onepage | url-status=live }}</ref><ref name="Stahl's Essential Psychopharmacology - dextroamphetamine">{{cite book | vauthors=Stahl SM | title=Prescriber's Guide: Stahl's Essential Psychopharmacology | date=March 2017 | publisher=Cambridge University Press | location=Cambridge, United Kingdom | isbn=9781108228749 | pages=39–44 | edition=6th | chapter-url=https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA39 | chapter=Amphetamine (D) | access-date=8 August 2017 }}</ref> |
|||
| Drugs.com = {{drugs.com|monograph|dextroamphetamine}} |
|||
| MedlinePlus = a605027 |
|||
| dependency_liability= Moderate-high<ref name="Stahl's Essential Psychopharmacology">{{cite book | vauthors=Stahl SM | title=Prescriber's Guide: Stahl's Essential Psychopharmacology | date=March 2017 | publisher=Cambridge University Press | location=Cambridge, United Kingdom | isbn=9781108228749 | pages=45–51 | edition=6th | chapter-url=https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA45#v=onepage | chapter=Amphetamine (D,L) | access-date=5 August 2017 | archive-date=8 June 2019 | archive-url=https://web.archive.org/web/20190608044902/https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA45#v=onepage | url-status=live }}</ref><ref name="Stahl's Essential Psychopharmacology - dextroamphetamine">{{cite book | vauthors=Stahl SM | title=Prescriber's Guide: Stahl's Essential Psychopharmacology | date=March 2017 | publisher=Cambridge University Press | location=Cambridge, United Kingdom | isbn=9781108228749 | pages=39–44 | edition=6th | chapter-url=https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA39 | chapter=Amphetamine (D) | access-date=8 August 2017 }}</ref> |
|||
| addiction_liability= Moderate-high<ref name="Stahl's Essential Psychopharmacology">{{cite book | vauthors=Stahl SM | title=Prescriber's Guide: Stahl's Essential Psychopharmacology | date=March 2017 | publisher=Cambridge University Press | location=Cambridge, United Kingdom | isbn=9781108228749 | pages=45–51 | edition=6th | chapter-url=https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA45#v=onepage | chapter=Amphetamine (D,L) | access-date=5 August 2017 | archive-date=8 June 2019 | archive-url=https://web.archive.org/web/20190608044902/https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA45#v=onepage | url-status=live }}</ref><ref name="Stahl's Essential Psychopharmacology - dextroamphetamine">{{cite book | vauthors=Stahl SM | title=Prescriber's Guide: Stahl's Essential Psychopharmacology | date=March 2017 | publisher=Cambridge University Press | location=Cambridge, United Kingdom | isbn=9781108228749 | pages=39–44 | edition=6th | chapter-url=https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA39 | chapter=Amphetamine (D) | access-date=8 August 2017 }}</ref> |
|||
<!-- Legal status --> |
|||
<!-- Legal status -->|legal_AU=S8|legal_CA=Schedule I|legal_DE=Anlage III|legal_UK=Class B|legal_US=Schedule II|legal_status=|licence_EU=|licence_US=Dextroamphetamine|bioavailability=By mouth: 75–100%<ref>{{cite encyclopedia | title=Dextromphetamine | url=http://www.drugbank.ca/drugs/DB01576#pharmacology | work=DrugBank | access-date=5 November 2013 | section=Pharmacology | archive-date=6 August 2019 | archive-url=https://web.archive.org/web/20190806163223/https://www.drugbank.ca/drugs/DB01576#pharmacology | url-status=live }}</ref>|protein_bound=15–40%<ref name="Drugbank-amph">{{cite encyclopedia | title=Amphetamine | section-url=http://www.drugbank.ca/drugs/DB00182#pharmacology | work=DrugBank | publisher=University of Alberta | access-date=5 November 2013 | date=8 February 2013 | section=Pharmacology | archive-date=12 October 2013 | archive-url=https://web.archive.org/web/20131012061802/http://www.drugbank.ca/drugs/DB00182#pharmacology | url-status=live }}</ref>|metabolism=[[CYP2D6]],<ref name="FDA Pharmacokinetics">{{cite web |title=HIGHLIGHTS OF PRESCRIBING INFORMATION |url=https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf |accessdate=28 December 2021 |archive-date=18 July 2018 |archive-url=https://web.archive.org/web/20180718220437/https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf |url-status=live }}</ref> [[Dopamine beta-hydroxylase|DBH]],<ref name="DBH ref">{{cite book | title=Foye's Principles of Medicinal Chemistry | year=2013 | publisher=Wolters Kluwer Health/Lippincott Williams & Wilkins | location=Philadelphia | isbn=978-1609133450 | page=648 |vauthors=Lemke TL, Williams DA, Roche VF, Zito W |edition=7th | quote=Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.}}</ref> [[Flavin-containing monooxygenase|FMO3]]<ref name="FMO">{{cite journal |last1=Krueger |first1=SK |last2=Williams |first2=DE |title=Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. |journal=Pharmacology & therapeutics |date=June 2005 |volume=106 |issue=3 |pages=357-87 |doi=10.1016/j.pharmthera.2005.01.001 |pmid=15922018}}</ref>|elimination_half-life=9–11 hours<ref name="FDA Pharmacokinetics">{{cite web |title=HIGHLIGHTS OF PRESCRIBING INFORMATION |url=https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf |accessdate=28 December 2021 |archive-date=18 July 2018 |archive-url=https://web.archive.org/web/20180718220437/https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf |url-status=live }}</ref><ref name="Adderall IR">{{cite web | title=Adderall IR Prescribing Information | url=http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/011522s042lbl.pdf | publisher=Teva Pharmaceuticals USA, Inc. | website=United States Food and Drug Administration | date=October 2015 | access-date=18 May 2016 | pages=1–6 | archive-date=15 September 2018 | archive-url=https://web.archive.org/web/20180915111231/https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/011522s042lbl.pdf | url-status=live }}</ref><br />[[pH]]-dependent: 7–34 hours<ref name="HSDB Toxnet October 2017 Full archived record" >{{cite web |title=AMPHETAMINE |url=https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/cgi-bin/sis/search2/f?.%2Ftemp%2F~mdjW95%3A1%3AFULL |accessdate=28 December 2021 |archive-date=2 October 2017 |archive-url=https://web.archive.org/web/20171002194327/https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/cgi-bin/sis/search2/f?.%2Ftemp%2F~mdjW95%3A1%3AFULL |url-status=bot: unknown }}</ref>|excretion=Kidney (45%);<ref>{{cite web|title=dextrostat (dextroamphetamine sulfate) tablet [Shire US Inc.]|publisher=Shire US Inc.|website=DailyMed|url=http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=1645|date=August 2006|access-date=8 November 2013|location=Wayne, PA|archive-date=13 January 2012|archive-url=https://web.archive.org/web/20120113020816/http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=1645|url-status=live}}</ref> urinary pH-dependent |
|||
| legal_AU = S8 |
|||
| legal_CA = Schedule I |
|||
| legal_DE = Anlage III |
|||
| legal_UK = Class B |
|||
| legal_US = Schedule II |
|||
| legal_status = |
|||
| licence_EU = |
|||
| licence_US = Dextroamphetamine |
|||
|quote = Table 21.2 Medications for ADHD ... D-amphetamine ... Onset: 30 min.</ref><ref>{{cite web|title = Dexedrine, ProCentra(dextroamphetamine) dosing, indications, interactions, adverse effects, and more|url = http://reference.medscape.com/drug/dexedrine-procentra-dextroamphetamine-342998#10|website = reference.medscape.com|access-date = 4 October 2015|quote = Onset of action: 1–1.5 hr|archive-date = 6 November 2018|archive-url = https://web.archive.org/web/20181106201922/https://reference.medscape.com/drug/dexedrine-procentra-dextroamphetamine-342998#10|url-status = live}}</ref><br />{{abbr|XR|Extended release}} dosing: 1.5–2 hours<ref name="Millichap: onset, peak, and duration">{{cite book | author = Millichap JG | editor = Millichap JG | title = Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD | year = 2010 | publisher = Springer | location = New York, USA | isbn = 9781441913968 | page = 112 | edition = 2nd | chapter = Chapter 9: Medications for ADHD | quote = <br />Table 9.2 Dextroamphetamine formulations of stimulant medication<br />Dexedrine [Peak:2–3 h] [Duration:5–6 h] ...<br />Adderall [Peak:2–3 h] [Duration:5–7 h]<br />Dexedrine spansules [Peak:7–8 h] [Duration:12 h] ...<br />Adderall XR [Peak:7–8 h] [Duration:12 h]<br />Vyvanse [Peak:3–4 h] [Duration:12 h]}}</ref><ref name="XR onset-duration">{{cite journal | vauthors = Brams M, Mao AR, Doyle RL | title = Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder | journal = Postgrad. Med. | volume = 120 | issue = 3 | pages = 69–88 | date = September 2008 | pmid = 18824827 | doi = 10.3810/pgm.2008.09.1909 | s2cid = 31791162 | quote = Onset of efficacy was earliest for d-MPH-ER at 0.5 hours, followed by d, l-MPH-LA at 1 to 2 hours, MCD at 1.5 hours, d, l-MPH-OR at 1 to 2 hours, MAS-XR at 1.5 to 2 hours, MTS at 2 hours, and LDX at approximately 2 hours. ... MAS-XR, and LDX have a long duration of action at 12 hours postdose}}</ref> |
|||
<!-- Pharmacokinetic data --> |
|||
<!-- Chemical data -->|C=9|H=13|N=1|SMILES=C[C@@H](Cc1ccccc1)N|StdInChI_Ref={{stdinchicite|changed|chemspider}}|StdInChI=InChI=1S/C9H13N/c1-8(10)7-9-5-3-2-4-6-9/h2-6,8H,7,10H2,1H3/t8-/m0/s1|StdInChI_comment=|StdInChIKey_Ref={{stdinchicite|correct|chemspider}}|StdInChIKey=KWTSXDURSIMDCE-QMMMGPOBSA-N|density=0.913|melting_point=|melting_notes=|boiling_point=201.5|boiling_notes=|solubility=20}} |
|||
| bioavailability = By mouth: 75–100%<ref>{{cite encyclopedia | title=Dextromphetamine | url=http://www.drugbank.ca/drugs/DB01576#pharmacology | work=DrugBank | access-date=5 November 2013 | section=Pharmacology | archive-date=6 August 2019 | archive-url=https://web.archive.org/web/20190806163223/https://www.drugbank.ca/drugs/DB01576#pharmacology | url-status=live }}</ref> |
|||
| protein_bound = 15–40%<ref name="Drugbank-amph">{{cite encyclopedia | title=Amphetamine | section-url=http://www.drugbank.ca/drugs/DB00182#pharmacology | work=DrugBank | publisher=University of Alberta | access-date=5 November 2013 | date=8 February 2013 | section=Pharmacology | archive-date=12 October 2013 | archive-url=https://web.archive.org/web/20131012061802/http://www.drugbank.ca/drugs/DB00182#pharmacology | url-status=live }}</ref> |
|||
| metabolism = [[CYP2D6]],<ref name="FDA Pharmacokinetics">{{cite web |title=HIGHLIGHTS OF PRESCRIBING INFORMATION |url=https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf |accessdate=28 December 2021 |archive-date=18 July 2018 |archive-url=https://web.archive.org/web/20180718220437/https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf |url-status=live }}</ref> [[Dopamine beta-hydroxylase|DBH]],<ref name="DBH ref">{{cite book | title=Foye's Principles of Medicinal Chemistry | year=2013 | publisher=Wolters Kluwer Health/Lippincott Williams & Wilkins | location=Philadelphia | isbn=978-1609133450 | page=648 |vauthors=Lemke TL, Williams DA, Roche VF, Zito W |edition=7th | quote=Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.}}</ref> [[Flavin-containing monooxygenase|FMO3]]<ref name="FMO">{{cite journal |last1=Krueger |first1=SK |last2=Williams |first2=DE |title=Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. |journal=Pharmacology & therapeutics |date=June 2005 |volume=106 |issue=3 |pages=357-87 |doi=10.1016/j.pharmthera.2005.01.001 |pmid=15922018}}</ref> |
|||
| elimination_half-life= 9–11 hours<ref name="FDA Pharmacokinetics">{{cite web |title=HIGHLIGHTS OF PRESCRIBING INFORMATION |url=https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf |accessdate=28 December 2021 |archive-date=18 July 2018 |archive-url=https://web.archive.org/web/20180718220437/https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf |url-status=live }}</ref><ref name="Adderall IR">{{cite web | title=Adderall IR Prescribing Information | url=http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/011522s042lbl.pdf | publisher=Teva Pharmaceuticals USA, Inc. | website=United States Food and Drug Administration | date=October 2015 | access-date=18 May 2016 | pages=1–6 | archive-date=15 September 2018 | archive-url=https://web.archive.org/web/20180915111231/https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/011522s042lbl.pdf | url-status=live }}</ref><br />[[pH]]-dependent: 7–34 hours<ref name="HSDB Toxnet October 2017 Full archived record" >{{cite web |title=AMPHETAMINE |url=https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/cgi-bin/sis/search2/f?.%2Ftemp%2F~mdjW95%3A1%3AFULL |accessdate=28 December 2021 |archive-date=2 October 2017 |archive-url=https://web.archive.org/web/20171002194327/https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/cgi-bin/sis/search2/f?.%2Ftemp%2F~mdjW95%3A1%3AFULL |url-status=bot: unknown }}</ref> |
|||
| excretion = Kidney (45%);<ref>{{cite web|title=dextrostat (dextroamphetamine sulfate) tablet [Shire US Inc.]|publisher=Shire US Inc.|website=DailyMed|url=http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=1645|date=August 2006|access-date=8 November 2013|location=Wayne, PA|archive-date=13 January 2012|archive-url=https://web.archive.org/web/20120113020816/http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=1645|url-status=live}}</ref> urinary pH-dependent |
|||
<!-- Chemical data --> |
|||
| ⚫ | '''デキストロアンフェタミン''' |
||
| C=9 | H=13 | N=1 |
|||
| SMILES = C[C@@H](Cc1ccccc1)N |
|||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
|||
| StdInChI = InChI=1S/C9H13N/c1-8(10)7-9-5-3-2-4-6-9/h2-6,8H,7,10H2,1H3/t8-/m0/s1 |
|||
| StdInChI_comment = |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = KWTSXDURSIMDCE-QMMMGPOBSA-N |
|||
| density = 0.913 |
|||
| melting_point = |
|||
| melting_notes = |
|||
| boiling_point = 201.5 |
|||
| boiling_notes = |
|||
| solubility = 20 |
|||
}} |
|||
<!-- Definition and medical uses --> |
|||
| ⚫ | '''デキストロアンフェタミン'''({{lang-en-short|Dextroamphetamine}}、'''D-AMP''')、または、'''デキサンアンフェタミン'''({{lang-en-short|dexamfetamine}})は、[[注意欠陥・多動性障害]](ADHD)や[[ナルコレプシー]]の治療に用いられる薬剤である<ref name="BNF81">{{Cite book |title=BNF 81: March-September 2021 |date=2021 |publisher=BMJ Group and the Pharmaceutical Press |isbn=978-0857114105 |page=370}}</ref>。短期間の[[肥満]]治療にも使用されることがあったが、肥満への使用は推奨されなくなった<ref name="AHFS2021">{{Cite web|title=Dextroamphetamine Monograph for Professionals |url=https://www.drugs.com/monograph/dextroamphetamine.html |website=Drugs.com |language=en |archive-date=11 August 2021 |archive-url=https://web.archive.org/web/20210811223953/https://www.drugs.com/monograph/dextroamphetamine.html |url-status=live |access-date=28 December 2021}}</ref>。投与法は経口である。仕事、運動、軍隊などでの能力向上に用いられることもあった<ref>{{Cite book |last=Miller |first=Richard Lawrence |title=The Encyclopedia of Addictive Drugs |date=2002 |publisher=Greenwood Publishing Group |isbn=978-0-313-31807-8 |page=107 |url=https://www.google.ca/books/edition/The_Encyclopedia_of_Addictive_Drugs/G7As-qawdzMC?hl=en&gbpv=1&dq=Dextroamphetamine+athletic&pg=PA107 |language=en |access-date=28 December 2021 |archive-date=11 January 2022 |archive-url=https://web.archive.org/web/20220111114548/https://www.google.ca/books/edition/The_Encyclopedia_of_Addictive_Drugs/G7As-qawdzMC?hl=en&gbpv=1&dq=Dextroamphetamine+athletic&pg=PA107 |url-status=live}}</ref>。 |
||
<!-- Side effects and mechanism --> |
|||
一般的な副作用には、腹痛、睡眠障害、[[動悸]]、高血圧、不安、震え、下痢などがあげられる<ref name="AHFS2021">{{Cite web|title=Dextroamphetamine Monograph for Professionals |url=https://www.drugs.com/monograph/dextroamphetamine.html |website=Drugs.com |language=en |archive-date=11 August 2021 |archive-url=https://web.archive.org/web/20210811223953/https://www.drugs.com/monograph/dextroamphetamine.html |url-status=live |access-date=28 December 2021}}</ref>。その他の副作用には、[[心筋症]]、虐待、[[精神病]]、攻撃性、発作などがあげられる。妊娠中の服用は未熟児を出産することがある。デキストロアンフェタミンは[[中枢神経系]](CNS)の[[精神刺激薬]]であり[[アンフェタミン]]の[[異性体|異生体]]である。 |
一般的な副作用には、腹痛、睡眠障害、[[動悸]]、高血圧、不安、震え、下痢などがあげられる<ref name="AHFS2021">{{Cite web|title=Dextroamphetamine Monograph for Professionals |url=https://www.drugs.com/monograph/dextroamphetamine.html |website=Drugs.com |language=en |archive-date=11 August 2021 |archive-url=https://web.archive.org/web/20210811223953/https://www.drugs.com/monograph/dextroamphetamine.html |url-status=live |access-date=28 December 2021}}</ref>。その他の副作用には、[[心筋症]]、虐待、[[精神病]]、攻撃性、発作などがあげられる。妊娠中の服用は未熟児を出産することがある。デキストロアンフェタミンは[[中枢神経系]](CNS)の[[精神刺激薬]]であり[[アンフェタミン]]の[[異性体|異生体]]である。 |
||
<!-- History and culture --> |
|||
デキストロアンフェタミンが医療薬として用いられるようになったのは1937年からである<ref>{{Cite book |last=Cutler |first=Janis |last2=Marcus |first2=Eric |title=Psychiatry |date=28 April 2010 |publisher=Oxford University Press |isbn=978-0-19-970682-2 |page=569 |url=https://www.google.ca/books/edition/Psychiatry/nMvqeG7lGwwC?hl=en&gbpv=1&dq=&pg=PA569 |language=en |access-date=28 December 2021 |archive-date=11 January 2022 |archive-url=https://web.archive.org/web/20220111114555/https://www.google.ca/books/edition/Psychiatry/nMvqeG7lGwwC?hl=en&gbpv=1&dq=&pg=PA569 |url-status=live}}</ref>。[[後発医薬品]]として入手できる<ref name="BNF81">{{Cite book |title=BNF 81: March-September 2021 |date=2021 |publisher=BMJ Group and the Pharmaceutical Press |isbn=978-0857114105 |page=370}}</ref>。2021年の英国の[[国民保健サービス]]にかかる費用は20mgの錠剤30錠で80ポンドほどである。米国では同じ量で60米ドルほどかかる<ref>{{Cite web |title=Dextroamphetamine Prices, Coupons & Savings Tips - GoodRx |url=https://www.goodrx.com/dextroamphetamine?dosage=20mg&form=tablet&label_override=dextroamphetamine&quantity=30&sort_type=popularity |website=GoodRx |access-date=28 December 2021}}</ref>。米国では[[規制物質法|規制物質法スケジュールII]]に定められている<ref name="PI2021">{{Cite web |title=DailyMed - DEXTROAMPHETAMINE SULFATE tablet |url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24163442-dd03-4066-a6ed-eb7e292b700b |website=dailymed.nlm.nih.gov |access-date=28 December 2021 |archive-date=11 January 2022 |archive-url=https://web.archive.org/web/20220111114550/https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24163442-dd03-4066-a6ed-eb7e292b700b |url-status=live}}</ref>。 |
デキストロアンフェタミンが医療薬として用いられるようになったのは1937年からである<ref>{{Cite book |last=Cutler |first=Janis |last2=Marcus |first2=Eric |title=Psychiatry |date=28 April 2010 |publisher=Oxford University Press |isbn=978-0-19-970682-2 |page=569 |url=https://www.google.ca/books/edition/Psychiatry/nMvqeG7lGwwC?hl=en&gbpv=1&dq=&pg=PA569 |language=en |access-date=28 December 2021 |archive-date=11 January 2022 |archive-url=https://web.archive.org/web/20220111114555/https://www.google.ca/books/edition/Psychiatry/nMvqeG7lGwwC?hl=en&gbpv=1&dq=&pg=PA569 |url-status=live}}</ref>。[[後発医薬品]]として入手できる<ref name="BNF81">{{Cite book |title=BNF 81: March-September 2021 |date=2021 |publisher=BMJ Group and the Pharmaceutical Press |isbn=978-0857114105 |page=370}}</ref>。2021年の英国の[[国民保健サービス]]にかかる費用は20mgの錠剤30錠で80ポンドほどである。米国では同じ量で60米ドルほどかかる<ref>{{Cite web |title=Dextroamphetamine Prices, Coupons & Savings Tips - GoodRx |url=https://www.goodrx.com/dextroamphetamine?dosage=20mg&form=tablet&label_override=dextroamphetamine&quantity=30&sort_type=popularity |website=GoodRx |access-date=28 December 2021}}</ref>。米国では[[規制物質法|規制物質法スケジュールII]]に定められている<ref name="PI2021">{{Cite web |title=DailyMed - DEXTROAMPHETAMINE SULFATE tablet |url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24163442-dd03-4066-a6ed-eb7e292b700b |website=dailymed.nlm.nih.gov |access-date=28 December 2021 |archive-date=11 January 2022 |archive-url=https://web.archive.org/web/20220111114550/https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24163442-dd03-4066-a6ed-eb7e292b700b |url-status=live}}</ref>。 |
||
== 出典 == |
== 出典 == |
||
<references /> |
<references /> |
||
[[Category:精神刺激薬]] |
|||
2023年7月3日 (月) 18:05時点における版
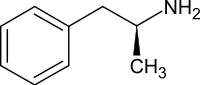 | |
| IUPAC命名法による物質名 | |
|---|---|
| |
| 臨床データ | |
| 発音 | [ˌdɛkstroʊæmˈfɛtəmiːn] |
| 販売名 | Dexedrine, DextroStat, Metamina, Attentin, Zenzedi, others |
| Drugs.com | monograph |
| MedlinePlus | a605027 |
| ライセンス | US FDA:リンク |
| 胎児危険度分類 | |
| 法的規制 |
|
| 依存性 | Moderate-high[1][2] |
| 嗜癖傾向 | Moderate-high[1][2] |
| 投与経路 | By mouth |
| 薬物動態データ | |
| 生物学的利用能 | By mouth: 75–100%[3] |
| 血漿タンパク結合 | 15–40%[4] |
| 代謝 | CYP2D6,[5] DBH,[6] FMO3[7] |
| 作用発現 | IR dosing: 0.5–1.5 hours[8] |
| 半減期 | 9–11 hours[5][9] pH-dependent: 7–34 hours[10] |
| 作用持続時間 | IR dosing: 3–6 hours[11][12] XR dosing: 8–12 hours[2][11][12] |
| 排泄 | Kidney (45%);[13] urinary pH-dependent |
| 識別 | |
| 別名 | D-amphetamine, dexamfetamine, dexamphetamine, (S)-amphetamine, (+)-amphetamine, dexamfetamine sulfate |
| 化学的データ | |
| 化学式 | C9H13N |
| 分子量 | 135.21 g·mol−1 |
| |
| 物理的データ | |
| 密度 | 0.913 g/cm3 |
| 沸点 | 201.5 °C (394.7 °F) |
| 水への溶解量 | 20 mg/mL (20 °C) |
デキストロアンフェタミン(英: Dextroamphetamine、D-AMP)、または、デキサンアンフェタミン(英: dexamfetamine)は、注意欠陥・多動性障害(ADHD)やナルコレプシーの治療に用いられる薬剤である[14]。短期間の肥満治療にも使用されることがあったが、肥満への使用は推奨されなくなった[15]。投与法は経口である。仕事、運動、軍隊などでの能力向上に用いられることもあった[16]。
一般的な副作用には、腹痛、睡眠障害、動悸、高血圧、不安、震え、下痢などがあげられる[15]。その他の副作用には、心筋症、虐待、精神病、攻撃性、発作などがあげられる。妊娠中の服用は未熟児を出産することがある。デキストロアンフェタミンは中枢神経系(CNS)の精神刺激薬でありアンフェタミンの異生体である。
デキストロアンフェタミンが医療薬として用いられるようになったのは1937年からである[17]。後発医薬品として入手できる[14]。2021年の英国の国民保健サービスにかかる費用は20mgの錠剤30錠で80ポンドほどである。米国では同じ量で60米ドルほどかかる[18]。米国では規制物質法スケジュールIIに定められている[19]。
出典
- ^ a b “Amphetamine (D,L)”. Prescriber's Guide: Stahl's Essential Psychopharmacology (6th ed.). Cambridge, United Kingdom: Cambridge University Press. (March 2017). pp. 45–51. ISBN 9781108228749. オリジナルの8 June 2019時点におけるアーカイブ。 2017年8月5日閲覧。
- ^ a b c “Amphetamine (D)”. Prescriber's Guide: Stahl's Essential Psychopharmacology (6th ed.). Cambridge, United Kingdom: Cambridge University Press. (March 2017). pp. 39–44. ISBN 9781108228749 2017年8月8日閲覧。
- ^ "Pharmacology". Dextromphetamine. 2019年8月6日時点のオリジナルよりアーカイブ。2013年11月5日閲覧。
{{cite encyclopedia}}:|work=は無視されます。 (説明) - ^ "Pharmacology". Amphetamine. 8 February 2013. 2013年10月12日時点のオリジナルよりアーカイブ。2013年11月5日閲覧。
{{cite encyclopedia}}:|work=は無視されます。 (説明) - ^ a b “HIGHLIGHTS OF PRESCRIBING INFORMATION”. 2018年7月18日時点のオリジナルよりアーカイブ。2021年12月28日閲覧。
- ^ Foye's Principles of Medicinal Chemistry (7th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. (2013). p. 648. ISBN 978-1609133450. "Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine."
- ^ Krueger, SK; Williams, DE (June 2005). “Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism.”. Pharmacology & therapeutics 106 (3): 357-87. doi:10.1016/j.pharmthera.2005.01.001. PMID 15922018.
- ^ Green-Hernandez, Carol; Singleton, Joanne K.; Aronzon, Daniel Z. (1 January 2001). Primary Care Pediatrics. Lippincott Williams & Wilkins. p. 243. ISBN 9780781720083. オリジナルの23 May 2016時点におけるアーカイブ。 2021年8月8日閲覧。
- ^ “Adderall IR Prescribing Information”. United States Food and Drug Administration. Teva Pharmaceuticals USA, Inc.. pp. 1–6 (2015年10月). 2018年9月15日時点のオリジナルよりアーカイブ。2016年5月18日閲覧。
- ^ “AMPHETAMINE”. 2017年10月2日時点のオリジナルよりアーカイブ。2021年12月28日閲覧。
- ^ a b 引用エラー: 無効な
<ref>タグです。「Millichap: onset, peak, and duration」という名前の注釈に対するテキストが指定されていません - ^ a b “A practical guide to the therapy of narcolepsy and hypersomnia syndromes”. Neurotherapeutics 9 (4): 739–752. (October 2012). doi:10.1007/s13311-012-0150-9. PMC 3480574. PMID 23065655.
- ^ “dextrostat (dextroamphetamine sulfate) tablet [Shire US Inc.]”. DailyMed. Wayne, PA: Shire US Inc. (2006年8月). 2012年1月13日時点のオリジナルよりアーカイブ。2013年11月8日閲覧。
- ^ a b BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. (2021). p. 370. ISBN 978-0857114105
- ^ a b “Dextroamphetamine Monograph for Professionals” (英語). Drugs.com. 2021年8月11日時点のオリジナルよりアーカイブ。2021年12月28日閲覧。
- ^ Miller, Richard Lawrence (2002) (英語). The Encyclopedia of Addictive Drugs. Greenwood Publishing Group. p. 107. ISBN 978-0-313-31807-8. オリジナルの11 January 2022時点におけるアーカイブ。 2021年12月28日閲覧。
- ^ Cutler, Janis; Marcus, Eric (28 April 2010) (英語). Psychiatry. Oxford University Press. p. 569. ISBN 978-0-19-970682-2. オリジナルの11 January 2022時点におけるアーカイブ。 2021年12月28日閲覧。
- ^ “Dextroamphetamine Prices, Coupons & Savings Tips - GoodRx”. GoodRx. 2021年12月28日閲覧。
- ^ “DailyMed - DEXTROAMPHETAMINE SULFATE tablet”. dailymed.nlm.nih.gov. 2022年1月11日時点のオリジナルよりアーカイブ。2021年12月28日閲覧。
