「閉塞性肺疾患」の版間の差分
編集の要約なし |
日本語版がプアなので英語版を順次訳します。協力願います |
||
| 1行目: | 1行目: | ||
{{翻訳中途|1=[http://en.wikipedia.org/w/index.php?title=Chronic_obstructive_pulmonary_disease&oldid=246754096 16:59, 21 October 2008]|date=2008年10月}}{{Medical}} |
|||
{{Medical}} |
|||
{{for|COPD occurring in horses|recurrent airway obstruction}} |
|||
'''閉塞性肺疾患'''(へいそくせいはいしっかん)とは、[[呼吸器疾患]]の一つで、[[気道]]の狭窄症状と[[肺]]の過膨張を主徴候とするものを指す。共通の所見として[[呼気延長]]、[[1秒率]]の低下、[[喘鳴]]、[[残気量]]の増加などが挙げられ、[[気管支喘息]]、[[慢性気管支炎]]、[[肺気腫]]、[[びまん性汎細気管支炎]]が含まれるとする。 |
|||
{{Infobox_Disease | |
|||
また最近では慢性気管支炎と肺気腫の2つを合わせて[[慢性閉塞性肺疾患]](COPD)という呼び方もされている。 |
|||
Name = Chronic Obstructive Pulmonary Disease | |
|||
なお、慢性閉塞性肺疾患に対する根治的治療法は現時点ではない。 |
|||
Image = | |
|||
主な発生起因は喫煙といわれており、COPD患者の90%は喫煙者である。 |
|||
Caption = | |
|||
DiseasesDB = 2672 | |
|||
ICD10 = {{ICD10|J|40||j|40}} - {{ICD10|J|44||j|40}}, {{ICD10|J|47||j|40}} | |
|||
ICD9 = {{ICD9|490}} - {{ICD9|492}}, {{ICD9|494}} - {{ICD9|496}} | |
|||
ICDO = | |
|||
OMIM = 606963 | |
|||
MedlinePlus = 000091 | |
|||
eMedicineSubj = med | |
|||
eMedicineTopic = 373 | |
|||
eMedicine_mult = {{eMedicine2|emerg|99}} | |
|||
MeshName = COPD | |
|||
MeshNumber = C08.381.495.389 | |
|||
}} |
|||
'''Chronic obstructive pulmonary disease''' ('''COPD''') is a [[disease]] of the lungs in which the [[airways]] become narrowed. This leads to a limitation of the flow of air to and from the lungs causing [[shortness of breath]]. In contrast to [[asthma]], the limitation of airflow is poorly reversible and usually gradually gets worse over time. |
|||
COPD is caused by noxious particles or gases, most commonly from [[smoking]], which trigger an abnormal [[inflammatory response]] in the lung.<ref name="pmid17507545">{{cite journal |author=Rabe KF, Hurd S, Anzueto A, ''et al'' |title=Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary |journal=Am. J. Respir. Crit. Care Med. |volume=176 |issue=6 |pages=532–55 |year=2007 |pmid=17507545 |doi=10.1164/rccm.200703-456SO}}</ref><ref name="Hogg">{{cite journal |author=Hogg JC, Chu F, Utokaparch S, ''et al''|journal=New England Journal of Medicine |volume=350 |issue=26 |pages=2645–53 |year=2004 |pmid=15215480 |
|||
| doi = 10.1056/NEJMoa032158 |
|||
| title = The Nature of Small-Airway Obstruction in Chronic Obstructive Pulmonary Disease}}</ref> The inflammatory response in the larger airways is known as [[chronic bronchitis]], which is diagnosed clinically when people regularly cough up [[sputum]]. In the [[alveoli]], the inflammatory response causes destruction of the tissue of the lung, a process known as [[emphysema]]. The natural course of COPD is characterized by occasional sudden worsenings of symptoms called acute exacerbations, most of which are caused by [[infections]] or [[air pollution]]. |
|||
The [[diagnosis]] of COPD requires [[lung function tests]]. Important management strategies are [[smoking cessation]], [[vaccinations]], [[rehabilitation]] and drug therapy (often using [[inhaler]]s). Some patients go on to requiring [[oxygen therapy|long-term oxygen therapy]] or [[lung transplantation]].<ref name="pmid17507545" /> |
|||
Worldwide, COPD ranked sixth as the cause of death in 1990. It is projected to be the third leading cause of death worldwide by 2020 due to an increase in smoking rates and demographic changes in many countries.<ref name="pmid17507545" /> COPD is the 4th leading cause of death in the U.S., and the economic burden of COPD in the U.S. in 2007 was $42.6 billion in health care costs and lost productivity.<ref>[http://www.nhlbi.nih.gov/health/public/lung/copd/ COPD (Chronic Obstructive Pulmonary Disease)<!-- Bot generated title -->]</ref><ref name = "NHLBI chart book">{{cite web |url= http://www.nhlbi.nih.gov/resources/docs/07a-chtbk.pdf |title= 2007 NHLBI Morbidity and Mortality Chart Book|accessdate=2008-06-06 |format= |work= }}</ref> |
|||
COPD is also known as '''chronic obstructive lung disease''' (COLD), '''chronic obstructive airway disease''' (COAD), '''chronic airflow limitation''' (CAL) and '''chronic obstructive respiratory disease'''. |
|||
==Signs and symptoms== |
|||
One of the most common [[symptoms]] of COPD is shortness of breath ([[dyspnea]]). People with COPD commonly describe this as: “My breathing requires effort”, “I feel out of breath” or “I can not get enough air in”.<ref name=”pmid16636091”>{{cite journal |author=Mahler DA|title=Mechanisms and measurement of dyspnea in chronic obstructive pulmonary disease |journal=Proceedings of the American Thoracic Society|volume=3 |issue=3 |pages=234–8|year=2006 |pmid=16636091|doi=10.1513/pats.200509-103SF}}</ref> People with COPD typically first notice dyspnea during vigorous exercise when the demands on the lungs are greatest. Over the years, dyspnea tends to get gradually worse so that it can occur during milder, everyday activities such as housework. In the advanced stages of COPD, dyspnea can become so bad that it occurs during rest and is constantly present. |
|||
Other symptoms of COPD are a persistent [[cough]], [[sputum]] or mucus production, [[wheezing]], chest tightness and tiredness.<ref>[http://www.nhlbi.nih.gov/health/dci/Diseases/Copd/Copd_SignsAndSymptoms.html U.S. National Heart Lung and Blood Institute - Signs and Symptoms]</ref><ref name="autogenerated1">[http://www.nlm.nih.gov/medlineplus/ency/article/000091.htm MedlinePlus Medical Encyclopedia: Chronic obstructive pulmonary disease<!-- Bot generated title -->]</ref> |
|||
People with advanced (very severe) COPD sometimes develop [[respiratory failure]]. When this happens, [[cyanosis]], a bluish discoloration of the lips caused by a lack of [[oxygen]] in the blood, can occur. An excess of carbon dioxide in the blood can cause headaches, drowsiness or twitching ([[asterixis]]). A complication of advanced COPD is [[cor pulmonale]], a strain on the heart due to the extra work required by the heart to pump blood through the affected lungs.<ref>[http://www.medicinenet.com/chronic_obstructive_pulmonary_disease_copd/page4.htm MedicineNet.com - COPD signs & symptoms]</ref> Symptoms of cor pulmonale are [[peripheral edema]], seen as swelling of the ankles, and dyspnea. |
|||
There are a few [[medical sign|signs]] of COPD that a healthcare worker may detect although they can be seen in other diseases. Some people have COPD and have none of these signs. Common signs are: |
|||
* [[tachypnea]], a rapid breathing rate |
|||
* wheezing sounds or crackles in the lungs heard through a [[stethoscope]] |
|||
* breathing out taking a longer time than breathing in |
|||
* enlargement of the chest, particularly the front-to-back distance ([[hyperinflation]]) |
|||
* active use of muscles in the neck to help with breathing |
|||
* breathing through pursed lips |
|||
==Etiology== |
|||
===Smoking=== |
|||
The primary risk factor for COPD is chronic tobacco smoking. In the [[United States]], 80 to 90% of cases of COPD are due to smoking.<ref name="medcauses">[http://www.medicinenet.com/chronic_obstructive_pulmonary_disease_copd/page3.htm7whatcauses MedicineNet.com - COPD causes]</ref> Exposure to cigarette smoke is measured in pack-years, the average number of packages of cigarettes smoked daily multiplied by the number of years of smoking. Not all smokers will develop COPD, but continuous smokers have at least a 25% risk after 25 years.<ref name="pmid17071833">{{cite journal |author=Løkke A, Lange P, Scharling H, Fabricius P, Vestbo J |title=Developing COPD: a 25 year follow up study of the general population |journal=Thorax |volume=61 |issue=11 |pages=935–9 |year=2006 |pmid=17071833 |doi=10.1136/thx.2006.062802}}</ref> The likelihood of developing COPD increases with increasing age as the cumulative smoke exposure increases. Inhaling the smoke from other peoples' cigarettes ([[passive smoking]]) can lead to impaired lung growth and could be a cause of COPD. |
|||
===Occupational exposures=== |
|||
Intense and prolonged exposure to workplace dusts found in [[coal mining]], [[gold mining]], and the cotton textile industry and chemicals such as [[cadmium]], [[isocyanates]], and fumes from [[welding]] have been implicated in the development of airflow obstruction, even in nonsmokers.<ref name="PMID16690673">{{cite journal |
|||
| pmid = 16690673 |
|||
| pmc = 1459603 |
|||
| title = ABC of chronic obstructive pulmonary disease. Definition, epidemiology, and risk factors |
|||
| year = 2006 |
|||
| journal = BMJ |
|||
| volume = 332 |
|||
| issue = 7550 |
|||
| pages = 1142–4 |
|||
| doi = 10.1136/bmj.332.7550.1142 |
|||
| month = May |
|||
| author = Devereux, Graham |
|||
}}</ref> Workers who smoke and are exposed to these particles and gases are even more likely to develop COPD. Intense [[silica]] dust exposure causes [[silicosis]], a restrictive lung disease distinct from COPD; however, less intense silica dust exposures have been linked to a COPD-like condition.<ref>{{cite journal |author=Hnizdo E, Vallyathan V |title=Chronic obstructive pulmonary disease due to occupational exposure to silica dust: a review of epidemiological and pathological evidence |journal=Occup Environ Med |volume=60 |issue=4 |pages=237–43 |year=2003 |month=April |pmid=12660371 |pmc=1740506 |doi= |url=}}</ref> The effect of occupational pollutants on the lungs appears to be substantially less important than the effect of cigarette smoking.<ref name="Harrisons">{{cite book |author=Loscalzo, Joseph; Fauci, Anthony S.; Braunwald, Eugene; Dennis L. Kasper; Hauser, Stephen L; Longo, Dan L. |title=Harrison's Principles of Internal Medicine |edition=17th Edition |publisher=McGraw-Hill Professional |location= |year=2008 |pages= |isbn=0-07-146633-9 |oclc= |doi= |accessdate=}}</ref> |
|||
===Air pollution=== |
|||
Studies in many countries have found that people who live in large cities have a higher rate of COPD compared to people who live in rural areas<ref>{{cite journal |author=Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM |title=Global burden of COPD: systematic review and meta-analysis |journal=Eur. Respir. J. |volume=28 |issue=3 |pages=523–32 |year=2006 |month=September |pmid=16611654 |doi=10.1183/09031936.06.00124605 |url=}}</ref>. Urban [[air pollution]] may be a contributing factor for COPD as it is thought to slow the normal growth of the lungs although the long-term research needed to confirm the link has not been done. In many [[developing countries]] indoor air pollution from cooking fire smoke (often using [[biomass fuel]]s such as wood and animal dung) is a common cause of COPD, especially in women<ref>{{cite journal |author=Kennedy SM, Chambers R, Du W, Dimich-Ward H |title=Environmental and occupational exposures: do they affect chronic obstructive pulmonary disease differently in women and men?|journal=Proceedings of the American Thoracic Society|volume=4 |issue=8 |pages=692–4 |year=2007 |month=December |pmid=18073405 |url=http://pats.atsjournals.org/cgi/content/full/4/8/692 |doi=10.1513/pats.200707-094SD}}</ref>. |
|||
===Genetics=== |
|||
Only about half of all long-term smokers will ever develop COPD. Some factor in addition to heavy smoke exposure is required for a person to develop COPD. This factor is probably a [[gene]]tic susceptibility. COPD is more common among relatives of COPD patients who smoke than unrelated smokers.<ref>{{cite journal |author=Silverman EK, Chapman HA, Drazen JM, ''et al'' |title=Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis |journal=Am. J. Respir. Crit. Care Med. |volume=157 |issue=6 Pt 1 |pages=1770–8 |year=1998 |month=June |pmid=9620904 |doi= |url=http://ajrccm.atsjournals.org/cgi/pmidlookup?view=long&pmid=9620904}}</ref> The genetic differences that make some peoples' lungs susceptible to the effects of tobacco smoke are mostly unknown. |
|||
[[Alpha 1-antitrypsin deficiency]] is a genetic condition that is responsible for about 2% of cases of COPD. In this condition, the body does not make enough of a protein, [[alpha 1-antitrypsin]]. Alpha 1-antitrypsin protects the lungs from damage caused by [[protease]] [[enzymes]], such as [[trypsin]], that can be released as a result of an inflammatory response to tobacco smoke.<ref>{{MedlinePlus|000091}}</ref> |
|||
===Other risk factors=== |
|||
A tendency to sudden airway constriction in response to inhaled irritants, bronchial hyperresponsiveness, is a characteristic of asthma. Many people with COPD also have this tendency. In COPD, the presence of bronchial hyperresponsiveness predicts a worse course of the disease.<ref name="Harrisons" /> It is not known if bronchial hyperresponsiveness is a cause or a consequence of COPD. |
|||
Other risk factors such as repeated lung [[infection]] and possibly a diet high in cured meats may be related to the development of COPD. |
|||
===COPD as an autoimmune disease=== |
|||
{{main|Autoimmunity}} |
|||
There is mounting evidence that there may be an autoimmune component to COPD. Many individuals with COPD who have stopped smoking have active inflammation in the lungs. The disease may continue to get worse for many years after stopping smoking due to this ongoing inflammation. This sustained inflammation is thought to be mediated by autoantibodies and autoreactive T cells.<ref>{{cite journal |author=Feghali-Bostwick CA, Gadgil AS, Otterbein LE, ''et al'' |title=Autoantibodies in patients with chronic obstructive pulmonary disease |journal=Am. J. Respir. Crit. Care Med. |volume=177 |issue=2 |pages=156–63 |year=2008 |month=January |pmid=17975205 |doi=10.1164/rccm.200701-014OC |url=}}</ref><ref>{{cite journal |author=Lee SH, Goswami S, Grudo A, ''et al'' |title=Antielastin autoimmunity in tobacco smoking-induced emphysema |journal=Nat. Med. |volume=13 |issue=5 |pages=567–9 |year=2007 |month=May |pmid=17450149 |doi=10.1038/nm1583 |url=}}</ref><ref>{{cite journal |
|||
| pmid = 10607796 |
|||
| pmc = PMC1745599 |
|||
| title = Ongoing airway inflammation in patients with COPD who do not currently smoke |
|||
| author = Rutgers, Steven R.; Postma, Dirkje S.; Ten Hacken, Nick H. .T.; Kauffman, Henk F.;van der Mark,Thomas W; Koeter, Gerard H.; Timens, Wim |
|||
| year = 2000 |
|||
| journal = Thorax |
|||
| volume = 55 |
|||
| issue = 1 |
|||
| pages = 12–18 |
|||
| doi = 10.1136/thorax.55.1.12 |
|||
}}</ref> |
|||
==Disease process== |
|||
[[Image:copd versus healthy lung.jpg|right|thumb|Enlarged view of lung tissue showing the difference between healthy lung and COPD]] |
|||
It is not fully understood how tobacco smoke and other inhaled particles damage the lungs to cause COPD. The most important processes causing lung damage are: |
|||
* [[Oxidative stress]] produced by the high concentrations of [[free radicals]] in tobacco smoke. |
|||
* [[Cytokine]] release due to [[inflammation]] as the body responds to irritant particles such as tobacco smoke in the airway. |
|||
* Tobacco smoke and free radicals impair the activity of antiprotease enzymes such as [[alpha 1-antitrypsin]], allowing [[protease]] enzymes to damage the lung. |
|||
===Pathology=== |
|||
====Chronic bronchitis==== |
|||
{{main|chronic bronchitis}} |
|||
Lung damage and inflammation in the large airways results in chronic bronchitis. Chronic bronchitis is defined in clinical terms as a cough with [[sputum]] production on most days for 3 months of a year, for 2 consecutive years<ref name="ohcm">{{cite book |author=Longmore, J. M.; Murray Longmore; Wilkinson, Ian; Supraj R. Rajagopalan |title=Oxford handbook of clinical medicine |publisher=Oxford University Press |location=Oxford [Oxfordshire] |year=2004 |pages=188-189 |isbn=0-19-852558-3 |oclc= |doi=}}</ref>. In the airways of the lung, |
|||
the hallmark of chronic bronchitris is an increased number ([[hyperplasia]]) and increased size ([[hypertrophy]]) of the [[goblet cells]] and [[mucous glands]] of the airway. As a result, there is more mucus than usual in the airways, contributing to narrowing of the airways and causing a cough with sputum. [[Microscope|Microscopically]] there is [[Infiltration (medical)|infiltration]] of the airway walls with [[Inflammation|inflammatory]] cells. Inflammation is followed by scarring and remodeling that thickens the walls and also results in narrowing of the airways. As chronic bronchitis progresses, there is [[squamous metaplasia]] (an abnormal change in the tissue lining the inside of the airway) and [[fibrosis]] (further thickening and scarring of the airway wall). The consequence of these changes is a limitation of airflow<ref name=kc>Kumar P, Clark M (2005). ''Clinical Medicine'', 6ed. Elsevier Saunders. pp 900-901. ISBN 0702027634.</ref>. |
|||
Patients suffering from COPD that present primarily chronic bronchitis rather than emphysema are commonly referred to as "blue bloaters" because of the bluish color of the skin and lips (cyanosis) seen in them. |
|||
====Emphysema==== |
|||
[[Image:Centrilobular emphysema 865 lores.jpg|thumb|[[Gross pathology]] of a lung showing centrilobular-type [[emphysema]] characteristic of smoking. This close-up of the [[Fixation (histology)|fixed]], cut lung surface shows multiple cavities lined by heavy black carbon deposits.]] |
|||
{{main|emphysema}} |
|||
Lung damage and inflammation of the air sacs ([[alveoli]]) results in emphysema. [[Emphysema]] is defined as enlargement of the air spaces [[distal]] to the [[terminal bronchioles]], with destruction of their walls.<ref name="ohcm"/> The destruction of air space walls reduces the [[surface area]] available for the exchange of [[oxygen]] and [[carbon dioxide]] during breathing. It also reduces the elasticity of the lung itself, which results in a loss of support for the airways that are embedded in the lung. These airways are more likely to collapse causing further limitation to airflow. |
|||
The effort made by patients suffering from emphysema during exhalation, causes a pink color in their faces, hence the term commonly used to refer to them, "pink puffers". |
|||
===Pathophysiology=== |
|||
Narrowing of the airways reduces the rate at which air can flow to and from the air sacs ([[alveoli]]) and limits the effectiveness of the lungs. In COPD, the greatest reduction in air flow occurs when breathing out (during expiration) because the pressure in the chest tends to compress rather than expand the airways. In theory, air flow could be increased by breathing more forcefully, increasing the pressure in the chest during expiration. In COPD, there is often a limit to how much this can actually increase air flow, a situation known as expiratory flow limitation<ref name="calverley">{{cite journal |author=Calverley PM, Koulouris NG |title=Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology|journal=Eur Respir J |volume=25 |pages=186-199|year=2005|pmid=15640341}}</ref>. |
|||
If the rate of airflow is too low, a person with COPD may not be able to completely finish breathing out (expiration) before he or she needs to take another breath. This is particularly common during exercise when breathing has to be faster. A little of the air of the previous breath remains within the lungs when the next breath is started. When this happens, there is an increase in the volume of air in the lungs, a process called dynamic hyperinflation.<ref name="calverley"/> |
|||
Dynamic hyperinflation is closely linked to shortness of breath ([[dyspnea]]) in COPD<ref name="o'donnell">{{cite journal |author=O'Donnell DE|title=Hyperinflation, Dyspnea, and Exercise Intolerance in Chronic Obstructive Pulmonary Disease|journal=The Proceedings of the American Thoracic Society|volume=3 |pages=180-184|year=2006|pmid=16565429}}</ref>. It is less comfortable to breathe with hyperinflation because it takes more effort to move the lungs and [[chest wall]] when they are already stretched by hyperinflation. |
|||
Another factor contributing to shortness of breath in COPD is the loss of the [[surface area]] available for the exchange of [[oxygen]] and [[carbon dioxide]] with emphysema. This reduces the rate of transfer of these gasses between the body and the atmosphere and can lead to low oxygen and high carbon dioxide levels in the body. A person with emphysema may have to breathe faster or more deeply to compensate, which can be difficult to do if there is also flow limitation or hyperinflation. |
|||
Some people with advanced COPD do manage to breathe fast to compensate, but usually have [[dyspnea]] as a result. Others, who may be less short of breath, tolerate low oxygen and high carbon dioxide levels in their bodies but this can eventually lead to headaches, drowsiness and heart failure. |
|||
Advanced COPD can lead to complications beyond the lungs such as weight loss ([[cachexia]]), [[pulmonary hypertension]] and heart failure ([[cor pulmonale]]). [[Osteoporosis]], [[heart disease]], [[muscle wasting]] and [[Major depressive disorder|depression]] are all more common in people with COPD<ref name="pmid17507545"/>. |
|||
===Acute exacerbations of COPD=== |
|||
An acute exacerbation of COPD is a sudden worsening of COPD symptoms (shortness of breath, quantity and color of phlegm) that typically lasts for several days. It may be triggered by an infection with bacteria or viruses or by environmental pollutants. Typically, infections cause 75% or more of the exacerbations; bacteria can roughly be found in 25% of cases, viruses in another 25%, and both viruses and bacteria in another 25%. Airway inflammation is increased during the exacerbation resulting in increased hyperinflation, reduced expiratory air flow and worsening of gas transfer.<ref name="pmid17507545"/> |
|||
== Diagnosis == |
|||
The diagnosis of COPD should be considered in anyone who has [[dyspnea]], chronic cough or sputum production, and/or a history of exposure to risk factors for the disease such as regular tobacco smoking<ref name="pmid17507545"/><ref name="pmid7956395">{{cite journal |author=Badgett RG, Tanaka DJ, Hunt DK, ''et al'' |title=The clinical evaluation for diagnosing obstructive airways disease in high-risk patients |journal=Chest |volume=106 |issue=5 |pages=1427–31 |year=1994 |pmid=7956395| doi = 10.1378/chest.106.5.1427}}</ref>. No single symptom or sign can adequately confirm or exclude the diagnosis of COPD<ref name="pmid7815660">{{cite journal |author=Holleman DR, Simel DL |title=Does the clinical examination predict airflow limitation? |journal=JAMA |volume=273 |issue=4 |pages=313–9 |year=1995 |pmid=7815660| doi = 10.1001/jama.273.4.313}}</ref> although COPD is uncommon under the age of 40 years. |
|||
===Spirometry=== |
|||
The diagnosis of COPD is confirmed by [[spirometry]]<ref name="pmid17507545"/>, a test that measures breathing. Spirometry measures the forced expiratory volume in one second (FEV<sub>1</sub>) which is the greatest volume of air that can be breathed out in the first second of a large breath. Spirometry also measures the forced vital capacity (FVC) which is the greatest volume of air that can be breathed out in a whole large breath. Normally at least 70% of the FVC comes out in the first second (i.e. the FEV<sub>1</sub>/FVC ratio is >70%). In COPD, this ratio is less than normal, (i.e. FEV<sub>1</sub>/FVC ratio is <70%) even after a [[bronchodilator]] medication has been given. |
|||
Spirometry can help to determine the severity of COPD.<ref name="pmid17507545"/> The FEV<sub>1</sub> (measured post-bronchodilator) is expressed as a percent of a predicted "normal" value based on a person's age, gender, height and weight: |
|||
{| class="wikitable" style="text-align:center;width:100%;" |
|||
|- |
|||
! Severity of COPD !! FEV<sub>1</sub> % predicted |
|||
|- |
|||
| Mild || ≥80 |
|||
|- |
|||
| Moderate || 50-79 |
|||
|- |
|||
| Severe || 30-49 |
|||
|- |
|||
| Very severe || <30 or Chronic respiratory failure symptoms |
|||
|} |
|||
The severity of COPD also depends on the severity of dyspnea and exercise limitation. These and other factors can be combined with spirometry results to obtain a COPD severity score that takes multiple dimensions of the disease into account<ref>{{cite journal |author=Celli BR, Cote CG, Marin JM, ''et al'' |title=The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease |journal=N. Engl. J. Med. |volume=350 |issue=10 |pages=1005–12 |year=2004 |month=March |pmid=14999112 |doi=10.1056/NEJMoa021322 |url=}}</ref>. |
|||
===Other tests=== |
|||
An x-ray of the chest may show an over-expanded lung (hyperinflation) and can be useful to help exclude other lung diseases. Complete pulmonary function tests with measurements of lung volumes and gas transfer may also show hyperinflation and can discriminate between COPD with emphysema and COPD without emphysema. A high-resolution [[computed tomography]] scan of the chest may show the distribution of emphysema throughout the lungs and can also be useful to exclude other lung diseases. |
|||
A blood sample taken from an [[artery]] can be tested for blood gas levels which may show low oxygen levels (hypoxemia) and/or high carbon dioxide levels (respiratory acidosis). A blood sample taken from a [[vein]] may show a high blood count (reactive polythycaemia), a reaction to long-term hypoxemia. |
|||
== Management == |
|||
There is currently no cure for COPD; however, COPD is both a preventable and treatable disease.<ref name="pmid17507545" /> [[Clinical practice guideline]]s for the management of COPD are available from the [[Global Initiative for Chronic Obstructive Lung Disease]] (GOLD),<ref>[http://www.goldcopd.org/ Global Initiative for Chronic Obstructive Lung Disease].</ref> a collaboration that includes the [[World Health Organization]] and the U.S. [[National Heart, Lung, and Blood Institute]]. For COPD exacerbations a meta analysis found that systemic corticosteroids were effective for all patients and that antibiotics and [[noninvasive positive pressure ventilation]] (NPPV) were effective for inpatients.<ref>{{cite journal |author=Quon BS, Gan WQ, Sin DD |title=Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis |journal=Chest |volume=133 |issue=3 |pages=756–66 |year=2008 |month=March |pmid=18321904 |doi=10.1378/chest.07-1207 |url=}}</ref> |
|||
===Smoking cessation=== |
|||
{{Main|Smoking cessation}} |
|||
Smoking cessation is one of the most important factors in slowing down the progression of COPD. Once COPD has been diagnosed, stopping smoking slows down the rate of progression of the disease. Even at a late stage of the disease it can significantly reduce the rate of deterioration in lung function and delay the onset of disability and death.<ref name=kc /> It is the only standard intervention that can improve the rate of progression of smoking-related COPD. |
|||
Smoking cessation starts with an individual decision to stop smoking that leads to an attempt at quitting. Often several attempts are required before long-term smoking cessation is achieved<ref name = "WHO">{{cite web|url=http://www.who.int/tobacco/resources/publications/tobacco_dependence/en/|title= World Health Organization Tobacco Free Initiative - Policy recommendations for smoking cessation and treatment of tobacco dependence| |
|||
accessdaymonth=28 July |accessyear=2008}} </ref>. Some smokers can achieve long-term smoking cessation through "willpower" alone. However smoking is highly addictive and many smokers need further support to quit. The chance of successfully stopping smoking can be greatly improved through social support, engagement in a smoking cessation programme and the use of drugs such as [[nicotine replacement therapy]], [[bupropion]] and [[varenicline]].<ref name="WHO"/> |
|||
The [[Tobacco smoking#Legal_issues_and_regulation|policies]] of governments, public health agencies and anti-smoking organizations can reduce smoking rates by encouraging smoking cessation and discouraging people from starting smoking.<ref name="WHO"/> These policies are important strategies in the prevention of COPD. |
|||
===Occupational health=== |
|||
Measures can be taken to reduce the likelihood that workers in at-risk industries such as coal mining will develop COPD. Some examples of these measures are: education of workers and management about the risks, promoting [[smoking cessation]], [[disease surveillance|surveillance]] of workers for early signs of COPD, the use of personal dust monitors, the use of [[respirators]] and dust control<ref>{{cite web |url=http://www.cdc.gov/niosh/mining/pubs/pdfs/2003-147.pdf |title=National Institute of Occupational Safety and Health - Handbook for Dust Control in Mining|accessmonthday= July 28|accessyear= 2008}}</ref>. Dust control can be achieved by improving ventilation, using water sprays and by using mining techniques that minimize dust generation. If a worker develops COPD, further lung damage can be reduced by avoiding ongoing dust exposure, for example by changing the work role. |
|||
===Environmental Change=== |
|||
Due to the individual differences in the causes and susceptibility to COPD, changes in the local environment of a COPD sufferer may have a significant and often subjectively subtle influence in the long term prognosis for the disease. Potential lung irritants such as common house dust, as well as such things as [[dust mites]] in bedding, (together with the rate at which bedding is changed/washed) as well as such domestic variables as carpets versus wooden floors, general or specific household dusts, cooking fumes etc, are all potential exacerbating influences in the progression of the disease. Sufferers are therefore strongly advised to discuss such factors with their health care provider. |
|||
===Pharmacotherapy=== |
|||
====Bronchodilators==== |
|||
There are several types of [[bronchodilators]] used clinically with varying efficacy: [[beta-2 adrenergic receptor|β<sub>2</sub>]] agonists, [[M3 receptor|M<sub>3</sub>]] antimuscarinics, leukotriene antagonists, cromones and xanthines.<ref name=thoracic>American Thoracic Society / European Respiratory Society Task Force (2005). ''Standards for the Diagnosis and Management of Patients with COPD''. Version 1.2. New York: American Thoracic Society. http://www.thoracic.org/go/copd</ref> These drugs relax the [[smooth muscles]] of the airway allowing for improved airflow. The change in [[FEV1|FEV<sub>1</sub>]] may not be substantial, but changes in the [[vital capacity]] are significant. Many patients feel less breathless after taking bronchodilators. |
|||
=====β<sub>2</sub> agonists===== |
|||
There are several highly specific β<sub>2</sub> agonists available. [[Salbutamol]] (Ventolin) is the most widely used short acting β<sub>2</sub> agonist to provide rapid relief and should be prescribed as a front line therapy for all classes of patients. Other β<sub>2</sub> agonists are [[Bambuterol]], [[Clenbuterol]], Fenoterol, and [[Formoterol]]. Long acting β<sub>2</sub> agonists (LABAs) such as [[Salmeterol]] act too slowly to be used as relief for [[dypsnea]] so these drugs should be used as maintenance therapy in the appropriate patient population. The TORCH study showed that LABA therapy reduced COPD exacerbation frequency over a 3 year period, compared to placebo.<ref name="pmid17314337">{{cite journal |author=Calverley PM, Anderson JA, Celli B, ''et al'' |title=Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease |journal=N. Engl. J. Med. |volume=356 |issue=8 |pages=775–89 |year=2007 |pmid=17314337 |doi=10.1056/NEJMoa063070}}</ref> An increased risk is associated with long acting β<sub>2</sub> agonists due to decreased sensitivity to inflammation so generally the use of a concomitant [[corticosteroid]] is indicated<ref>{{cite web |url=http://www.fda.gov/medwatch/SAFETY/2003/serevent.htm |title=MedWatch - Serevent Dear Healthcare Professional letter |format= |work= |accessdate=}}</ref><ref>{{cite web |url=http://www.gsk.com/press_archive/press2003/press_01232003.htm |title=GlaxoSmithKline - Press Archive GSK announces interim US Serevent study results |format= |work= |accessdate=}}</ref><ref>{{cite web |url=http://www.medscape.com/viewarticle/527629_print |title=Medscape 527629 - please fix|format= |work= |accessdate=}}</ref>. |
|||
=====M<sub>3</sub> muscarinic antagonists (anticholinergics)===== |
|||
M<sub>3</sub> muscarinic antagonists are anticholinergics. Specific [[antimuscarinics]] were found to provide effective relief to COPD. Inhaled antimuscarinics have the advantage of avoiding [[endocrine]] and [[exocrine]] M<sub>3</sub> receptors. The quaternary M<sub>3</sub> muscarinic antagonist [[Ipratropium]] is widely prescribed with the β<sub>2</sub> agonist [[salbutamol]]. |
|||
[http://www.neurosci.pharm.utoledo.edu/MBC3320/muscarinic.htm]. Ipratropium formerly was offered combined with salbutamol (Combivent) and with fenoterol (Duovent) but due to the CFC propellant, these products have been withdrawn. |
|||
[[Tiotropium]] provides improved specificity for M<sub>3</sub> muscarinic receptors. It is a long acting muscarinic antagonist that has shown good efficacy in the reduction of exacerbations of COPD, especially when combined with a LABA and inhaled steroid.<ref name="pmid17310045">{{cite journal |author=Aaron SD, Vandemheen KL, Fergusson D, ''et al'' |title=Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial |journal=Ann. Intern. Med. |volume=146 |issue=8 |pages=545–55 |year=2007 |pmid=17310045 |doi=}}</ref> |
|||
=====Cromones===== |
|||
[[Cromone]]s are [[mast cell stabilizer]]s that are thought to act on a [[chloride channel]] found on [[mast cells]] that help reduce the production of [[histamine]] and other inflammatory factors. Chromones are also thought to act on IgE-regulated [[calcium channels]] on mast cells. [[Cromoglicate]] and [[Nedocromil]], which has a longer half-life, are two chromones available.<ref name="pmid4166895">{{cite journal |author=Howell JB, Altounyan RE |title=A double-blind trial of disodium cromoglycate in the treatment of allergic bronchial asthma |journal=Lancet |volume=2 |issue=7515 |pages=539–42 |year=1967 |pmid=4166895 |doi=}}</ref> |
|||
=====Leukotriene antagonists===== |
|||
More recently [[leukotriene]] antagonists block the signalling molecules used by the immune system. [[Montelukast]], [[Pranlukast]], [[Zafirlukast]] are some of the leukotrienes antagonists.<ref name="pmid13804592">{{cite journal |author=BROCKLEHURST WE |title=The release of histamine and formation of a slow-reacting substance (SRS-A) during anaphylactic shock |journal=J. Physiol. (Lond.) |volume=151 |issue= |pages=416–35 |year=1960 |pmid=13804592 |doi=}}</ref>These agents have not been tested in good, controlled trials, <ref>From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2007. Available from: http://www.goldcopd.org. |
|||
</ref> and as such, there is no data to support the use of these agents in COPD.<ref>Standards for the Diagnosis and Management of Patients with COPD, American Thoracic Society and the European Respiratory Society 2005. Available from: http://www.thoracic.org/sections/copd/resources/copddoc.pdf. |
|||
</ref> |
|||
=====Xanthines===== |
|||
[[Theophylline]] is the prototype of the [[xanthine]]<!-- <ref>http://www.chemistry.org/portal/a/c/s/1/acsdisplay.html?DOC=HomeMolecule/archive/motw_xanthine_arch.html</ref> --> class of drug. Teas are natural sources of methylxanthines, xanthines and [[caffeine]] while [[cocoa]] is a natural source of [[theobromine]]. [[Caffeine]] is approximately 16% metabolized into theophylline. Nebulized theophylline is used in the EMR for treatment of [[dyspnea]] (Difficulty in breathing). Patients need continual monitoring as theophylline has a narrow [[therapeutic range]]. More aggressive EMR interventions include IV H<sub>1</sub> [[antihistamine]]s and IM [[dexamethasone]]. |
|||
[[Theophylline]] antagonizes [[phosphodiesterase]], and small reductions in COPD exacerbation rates have been demonstrated.<ref name="pmid16916334">{{cite journal |author=Zhou Y, Wang X, Zeng X, ''et al'' |title=Positive benefits of theophylline in a randomized, double-blind, parallel-group, placebo-controlled study of low-dose, slow-release theophylline in the treatment of COPD for 1 year |journal=Respirology |volume=11 |issue=5 |pages=603–10 |year=2006 |pmid=16916334 |doi=10.1111/j.1440-1843.2006.00897.x}}</ref> The investigative phosphodiesterase-4 antagonists, [[roflumilast]] and [[cilomilast]] have completed Phase-2 clinical trials. |
|||
====Corticosteroids==== |
|||
Enteral and parenteral corticosteroid therapy has long been the mainstay of treatment of COPD, and is known to reduce length of stay in hospital. Similarly, inhaled [[corticosteroids]] (specifically [[glucocorticoids]]) act in the inflammatory cascade and improve airway function considerably,<ref name=kc /> and have been shown in the ISOLDE trial to reduce the number of COPD exacerbations by 25%.<ref name="pmid10807619">{{cite journal |author=Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK |title=Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial |journal=BMJ |volume=320 |issue=7245 |pages=1297–303 |year=2000 |pmid=10807619 | doi = 10.1136/bmj.320.7245.1297|url=http://www.bmj.com/cgi/content/full/320/7245/1297}}</ref> Corticosteroids are often combined with bronchodilators in a single inhaler. Some of the more common inhaled steroids in use are [[beclomethasone]], [[Mometasone furoate|mometasone]], and [[fluticasone]]. |
|||
Salmeterol and fluticasone are combined (Advair), however the reduction in death from all causes among patients with COPD in the combination therapy group did not reach the predetermined level of statistical significance.<ref>{{cite journal |author=Calverley PM, Anderson JA, Celli B, ''et al'' |title=Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease |journal=N. Engl. J. Med. |volume=356 |issue=8 |pages=775–89 |year=2007 |month=February |pmid=17314337 |doi=10.1056/NEJMoa063070 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=17314337&promo=ONFLNS19}}</ref><ref>[http://clinicaltrials.gov/show/NCT00268216 Survival Of Subjects With Chronic Obstructive Pulmonary Disease (COPD) - Full Text View - ClinicalTrials.gov<!-- Bot generated title -->]</ref> |
|||
====TNF antagonists==== |
|||
Tumor necrosis factor antagonists (TNF) are the most recent class of medications designed to deal with refractory cases. [[Tumor necrosis factor-alpha]] is a cachexin or cachectin and is considered a so-called biological drug. They are considered immunosopressive with attendant risks. These rather expensive drugs include [[infliximab]], [[adalimumab]] and [[etanercept]].<ref>[http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/C/CellSignaling.html Cell Signaling<!-- Bot generated title -->]</ref>Infliximab has been trialled in COPD with no evidence of benefit, with the possibility of harm. This was a relatively small study (77-79 patients in each arm).<ref>{{cite journal |author=Rennard SI, Fogarty C, Kelsen S, ''et al'' |title=The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease |journal=Am. J. Respir. Crit. Care Med. |volume=175 |issue=9 |pages=926–34 |year=2007 |month=May |pmid=17290043 |doi=10.1164/rccm.200607-995OC |url=}}</ref> |
|||
====Supplemental oxygen==== |
|||
[[Image:Home oxygen concentrator.jpg|thumb|Oxygen can be delivered in different forms: in large containers, in smaller containers with [[liquid oxygen]], or with the use of a [[oxygen concentrator]] (''shown here'') which derives oxygen from room air. The latter two options improve mobility of people requiring long-term oxygen therapy.]] |
|||
In general, [[oxygen therapy|long-term oxygen therapy]] is reserved for individuals with COPD who have arterial [[hypoxemia]] ([[PaO2]] less than 55 mm Hg), or a PaO2 between 55 and 60 mm Hg with evidence of [[pulmonary hypertension]], [[cor pulmonale]], or secondary [[erythrocytosis]] (hematocrit >55%). In these patients, continuous home oxygen therapy (for >15 h/d) sufficient to correct hypoxemia has been shown to improve survival. <ref>{{cite journal |author= |title=Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party |journal=Lancet |volume=1 |issue=8222 |pages=681–6 |year=1981 |month=March |pmid=6110912 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(81)91970-X}}</ref> The use of low flow oxygen may be necessary in some patients because in the COPD patient, [[control of respiration]] is driven mainly by the blood oxygen level rather than the carbon dioxide level, increased oxygen delivery can diminish this response and cause respiratory failure. |
|||
====Vaccination==== |
|||
Patients with COPD should be routinely [[vaccination|vaccinated]] against [[influenza]] (yearly), [[pneumococcus]] (every 5 years) and other diseases to prevent illness and reduce the possibility of death.<ref name="thoracic" /> |
|||
====Pulmonary rehabilitation==== |
|||
Pulmonary rehabilitation is a program of disease management, counseling and exercise coordinated to benefit the individual.<ref>[http://www.nhlbi.nih.gov/health/dci/Diseases/Copd/Copd_Treatments.html U.S. National Heart Lung and Blood Institute - Treatment]</ref> Pulmonary rehabilitation has been shown to relieve difficulties breathing and fatigue. It has also been shown to improve the sense of control a patient has over their disease as well as their emotions.<ref name="pmid12137716">{{cite journal |author=Lacasse Y, Brosseau L, Milne S, ''et al'' |title=Pulmonary rehabilitation for chronic obstructive pulmonary disease |journal=Cochrane database of systematic reviews (Online) |volume= |issue=3 |pages=CD003793 |year=2002 |pmid=12137716 |doi=}}</ref> |
|||
===Diet=== |
|||
A recent French study conducted over 12 years with almost 43,000 men concluded that eating a [[Mediterranean diet]] "halves the risk of serious lung disease like emphysema and bronchitis". <ref>{{cite web |url=http://news.bbc.co.uk/2/hi/health/6647811.stm |title=BBC NEWS Health Med diet 'cuts lung disease risk' |format= |work= |accessdate=}}</ref><ref>{{cite journal |author=Aniwidyaningsih W, Varraso R, Cano N, Pison C |title=Impact of nutritional status on body functioning in chronic obstructive pulmonary disease and how to intervene |journal=Curr Opin Clin Nutr Metab Care |volume=11 |issue=4 |pages=435–442 |year=2008 |month=July |pmid=18542004 |doi=10.1097/MCO.0b013e3283023d37 |url= |doi_brokendate=2008-07-06}}</ref> |
|||
==Treatment== |
|||
Treatment for COPD includes inhalers that dilate the airways (bronchodilators) and sometimes theophylline. The COPD patient must stop smoking. In some cases inhaled steroids are used to suppress lung inflammation and, in severe cases or flare-ups, intravenous or oral steroids are given. |
|||
Antibiotics are used during flare-ups of symptoms as infections can cause acute exacerbations of COPD, though they have been shown to have only a small effect.<ref>Gibson, et al. ''Evidence-based Respiratory Medicine''. Blackwell Publishing, 2005. ISBN 072791605X. [http://books.google.ca/books?id=mobHvIEVlsMC&pg=PA390&lpg=PA390&dq=antibiotics+copd&source=web&ots=u-9MNcI_NV&sig=Qx0PJB2Al3H2BDt4xYvY0QympLc&hl=en&sa=X&oi=book_result&resnum=4&ct=result#PPA392,M1 pp. 390-392.]</ref> Chronic, low-flow oxygen, non-invasive ventilation, or intubation may be needed in some cases. Surgery to remove parts of the disease lung has been shown to be helpful for some patients with COPD.{{Fact|date=February 2008}} |
|||
Lung rehabilitation programs may help some patients. |
|||
Lung transplant is sometimes performed for severe cases (preferentially for younger sufferers in many first world countries). |
|||
===Support groups=== |
|||
The stress of illness can often be helped by joining a support group where members share common experiences and problems. |
|||
<ref name="autogenerated1" /> |
|||
==Prognosis== |
|||
A good prognosis of COPD relies on an early diagnosis and prompt treatment. Most patients will have improvement in lung function once treatment is started, however eventually signs and symptoms will worsen as COPD progresses. The median survival is about 10 years if two-thirds of expected lung function was lost by diagnosis. |
|||
"End Stages" of COPD are diagnosed as the normal ratio of carbon dioxide being inordinately higher than the volume of oxygen in the bloodstream. Although end stage COPD may mean death is imminent in cases dealing with degenerative diseases, forced oxygen therapy and other treatments may successfully be used to prolong life but have not been proven to be successful for the long run. These types of therapy may be successfully used in COPD cases caused by curable diseases such as bronchitis. |
|||
===Bronchitis=== |
|||
Acute [[bronchitis]] usually resolves in 2-10 years. |
|||
===Emphysema=== |
|||
The outcome is better for patients with less damage to the lungs who stop smoking any substance immediately (a fact that cannot be over emphasised). Still, patients with extensive lung damage may live for many years, so predicting prognosis is very difficult. Death may occur from respiratory failure, [[pneumonia]], or other complications, such as influenza. |
|||
===Pneumoconiosis=== |
|||
The outcome is good for patients with minimal damage to the lung. However, patients with extensive lung damage may live for many years so predicting prognosis is difficult. Death may occur from [[respiratory failure]], [[pneumonia]], [[cor pulmonale]] or other complications. |
|||
===Pulmonary neoplasms=== |
|||
The stage of the [[tumor]](s) has a major impact on [[neoplasm]] prognosis. Staging is the process of determining tumor size, growth rate, potential [[metastasis]], lymph node involvement, treatment options and prognosis. Two-year prognosis for limited small cell pulmonary neoplasms is twenty percent and for extensive disease five percent. The average life expectancy for someone with recurrent small cell pulmonary neoplasms is two to three months.<ref>{{cite web |url=http://www.lungdiseasefocus.com/lung-cancer/cancer-prognosis.php |title=Site Map: Lung.com |format= |work= |accessdate=}}</ref> |
|||
The 5-year overall survival rate for pulmonary neoplasms is 14%.<ref>{{cite book |author=Jameson, J. N. St C.; Dennis L. Kasper; Harrison, Tinsley Randolph; Braunwald, Eugene; Fauci, Anthony S.; Hauser, Stephen L; Longo, Dan L. |title=Harrison's principles of internal medicine |publisher=McGraw-Hill Medical Publishing Division |location=New York |year=2005 |pages=506| chapter=John D. Minna, Neoplasms of the Lung |isbn=0-07-140235-7 |oclc= |doi= |accessdate=}}</ref> |
|||
== Epidemiology == |
|||
According to the [[World Health Organization]] (WHO), 80 million people suffer from moderate to severe COPD and 3 million died due to it in 2005. The WHO predicts that by 2030, it will be the 3rd largest cause of mortality worldwide.<ref>[http://www.who.int/respiratory/copd/en/ WHO - COPD]</ref> |
|||
Since COPD is not diagnosed until it becomes clinically apparent, prevalence and mortality data greatly underestimate the [[Socioeconomics|socioeconomic]] burden of COPD.<ref name=thoracic /> In the UK, COPD accounts for about 7% of all days of sickness related absence from work.<ref name=kc /> |
|||
Smoking rates in the industrialized world have continued to fall, causing rates of emphysema and pulmonary neoplasms to slowly decline. |
|||
==Footnotes== |
|||
{{reflist|2}} |
|||
== External links == |
|||
*[http://www.learnaboutcopd.org National Heart, Lung and Blood Institute - COPD] U.S. NHLBI Information for Patients and the Public page. |
|||
*[http://crd.sagepub.com Chronic Respiratory Disease - leading research and articles on respiratory disease] |
|||
*[http://www.goldcopd.com Global Initiative for Chronic Obstructive Lung Disease (GOLD)] |
|||
*[http://www.copd.about.com Chronic Obstructive Pulmonary Disease - medical information and informative articles on COPD] |
|||
{{Respiratory pathology}} |
|||
[[Category:Aging-associated diseases]] |
|||
[[Category:Occupational diseases]] |
|||
[[Category:pulmonology]] |
|||
{{Link FA|pl}} |
|||
[[ar:انسداد شعب هوائية مزمن]] |
|||
[[ca:Malaltia pulmonar obstructiva crònica]] |
|||
[[da:Kronisk Obstruktiv Lungesygdom]] |
|||
[[de:Chronisch obstruktive Lungenerkrankung]] |
|||
[[es:Enfermedad Pulmonar Obstructiva Crónica]] |
|||
[[eu:BGBK]] |
|||
[[fr:Broncho-pneumopathie chronique obstructive]] |
|||
[[hr:Kronična opstruktivna plućna bolest]] |
|||
[[id:Penyakit paru obstruktif kronik]] |
|||
[[it:Bronchite cronica]] |
|||
[[he:מחלת ריאות חסימתית כרונית]] |
|||
[[nl:COPD]] |
|||
[[ja:慢性閉塞性肺疾患]] |
|||
[[no:Kronisk obstruktiv lungesykdom]] |
|||
[[nn:Kronisk obstruktiv lungesjukdom]] |
|||
[[pl:Przewlekła obturacyjna choroba płuc]] |
|||
[[pt:DPOC]] |
|||
[[ru:Хроническая обструктивная болезнь лёгких]] |
|||
[[sr:Хронична опструктивна болест плућа]] |
|||
[[fi:Keuhkoahtaumatauti]] |
|||
[[sv:Kronisk obstruktiv lungsjukdom]] |
|||
[[vi:Bệnh phổi tắc nghẽn mạn tính]] |
|||
[[ur:مزمن مسدودی پھیپڑی مرض]] |
|||
[[zh:慢性阻塞性肺病]] |
|||
---- |
|||
[[Category:呼吸器疾患|へいそくせいはいしつかん]] |
[[Category:呼吸器疾患|へいそくせいはいしつかん]] |
||
2008年10月23日 (木) 16:35時点における版
この項目「閉塞性肺疾患」は途中まで翻訳されたものです。(原文:16:59, 21 October 2008) 翻訳作業に協力して下さる方を求めています。ノートページや履歴、翻訳のガイドラインも参照してください。要約欄への翻訳情報の記入をお忘れなく。(2008年10月) |
ウィキペディアは医学的助言を提供しません。免責事項もお読みください。 |
| 閉塞性肺疾患 | |
|---|---|
| 概要 | |
| 診療科 | 呼吸器学 |
| 分類および外部参照情報 | |
| ICD-10 | J40 - J44, J47 |
| ICD-9-CM | 490 - 492, 494 - 496 |
| OMIM | 606963 |
| DiseasesDB | 2672 |
| MedlinePlus | 000091 |
| eMedicine | med/373 emerg/99 |
Chronic obstructive pulmonary disease (COPD) is a disease of the lungs in which the airways become narrowed. This leads to a limitation of the flow of air to and from the lungs causing shortness of breath. In contrast to asthma, the limitation of airflow is poorly reversible and usually gradually gets worse over time.
COPD is caused by noxious particles or gases, most commonly from smoking, which trigger an abnormal inflammatory response in the lung.[1][2] The inflammatory response in the larger airways is known as chronic bronchitis, which is diagnosed clinically when people regularly cough up sputum. In the alveoli, the inflammatory response causes destruction of the tissue of the lung, a process known as emphysema. The natural course of COPD is characterized by occasional sudden worsenings of symptoms called acute exacerbations, most of which are caused by infections or air pollution.
The diagnosis of COPD requires lung function tests. Important management strategies are smoking cessation, vaccinations, rehabilitation and drug therapy (often using inhalers). Some patients go on to requiring long-term oxygen therapy or lung transplantation.[1]
Worldwide, COPD ranked sixth as the cause of death in 1990. It is projected to be the third leading cause of death worldwide by 2020 due to an increase in smoking rates and demographic changes in many countries.[1] COPD is the 4th leading cause of death in the U.S., and the economic burden of COPD in the U.S. in 2007 was $42.6 billion in health care costs and lost productivity.[3][4]
COPD is also known as chronic obstructive lung disease (COLD), chronic obstructive airway disease (COAD), chronic airflow limitation (CAL) and chronic obstructive respiratory disease.
Signs and symptoms
One of the most common symptoms of COPD is shortness of breath (dyspnea). People with COPD commonly describe this as: “My breathing requires effort”, “I feel out of breath” or “I can not get enough air in”.[5] People with COPD typically first notice dyspnea during vigorous exercise when the demands on the lungs are greatest. Over the years, dyspnea tends to get gradually worse so that it can occur during milder, everyday activities such as housework. In the advanced stages of COPD, dyspnea can become so bad that it occurs during rest and is constantly present. Other symptoms of COPD are a persistent cough, sputum or mucus production, wheezing, chest tightness and tiredness.[6][7] People with advanced (very severe) COPD sometimes develop respiratory failure. When this happens, cyanosis, a bluish discoloration of the lips caused by a lack of oxygen in the blood, can occur. An excess of carbon dioxide in the blood can cause headaches, drowsiness or twitching (asterixis). A complication of advanced COPD is cor pulmonale, a strain on the heart due to the extra work required by the heart to pump blood through the affected lungs.[8] Symptoms of cor pulmonale are peripheral edema, seen as swelling of the ankles, and dyspnea.
There are a few signs of COPD that a healthcare worker may detect although they can be seen in other diseases. Some people have COPD and have none of these signs. Common signs are:
- tachypnea, a rapid breathing rate
- wheezing sounds or crackles in the lungs heard through a stethoscope
- breathing out taking a longer time than breathing in
- enlargement of the chest, particularly the front-to-back distance (hyperinflation)
- active use of muscles in the neck to help with breathing
- breathing through pursed lips
Etiology
Smoking
The primary risk factor for COPD is chronic tobacco smoking. In the United States, 80 to 90% of cases of COPD are due to smoking.[9] Exposure to cigarette smoke is measured in pack-years, the average number of packages of cigarettes smoked daily multiplied by the number of years of smoking. Not all smokers will develop COPD, but continuous smokers have at least a 25% risk after 25 years.[10] The likelihood of developing COPD increases with increasing age as the cumulative smoke exposure increases. Inhaling the smoke from other peoples' cigarettes (passive smoking) can lead to impaired lung growth and could be a cause of COPD.
Occupational exposures
Intense and prolonged exposure to workplace dusts found in coal mining, gold mining, and the cotton textile industry and chemicals such as cadmium, isocyanates, and fumes from welding have been implicated in the development of airflow obstruction, even in nonsmokers.[11] Workers who smoke and are exposed to these particles and gases are even more likely to develop COPD. Intense silica dust exposure causes silicosis, a restrictive lung disease distinct from COPD; however, less intense silica dust exposures have been linked to a COPD-like condition.[12] The effect of occupational pollutants on the lungs appears to be substantially less important than the effect of cigarette smoking.[13]
Air pollution
Studies in many countries have found that people who live in large cities have a higher rate of COPD compared to people who live in rural areas[14]. Urban air pollution may be a contributing factor for COPD as it is thought to slow the normal growth of the lungs although the long-term research needed to confirm the link has not been done. In many developing countries indoor air pollution from cooking fire smoke (often using biomass fuels such as wood and animal dung) is a common cause of COPD, especially in women[15].
Genetics
Only about half of all long-term smokers will ever develop COPD. Some factor in addition to heavy smoke exposure is required for a person to develop COPD. This factor is probably a genetic susceptibility. COPD is more common among relatives of COPD patients who smoke than unrelated smokers.[16] The genetic differences that make some peoples' lungs susceptible to the effects of tobacco smoke are mostly unknown. Alpha 1-antitrypsin deficiency is a genetic condition that is responsible for about 2% of cases of COPD. In this condition, the body does not make enough of a protein, alpha 1-antitrypsin. Alpha 1-antitrypsin protects the lungs from damage caused by protease enzymes, such as trypsin, that can be released as a result of an inflammatory response to tobacco smoke.[17]
Other risk factors
A tendency to sudden airway constriction in response to inhaled irritants, bronchial hyperresponsiveness, is a characteristic of asthma. Many people with COPD also have this tendency. In COPD, the presence of bronchial hyperresponsiveness predicts a worse course of the disease.[13] It is not known if bronchial hyperresponsiveness is a cause or a consequence of COPD. Other risk factors such as repeated lung infection and possibly a diet high in cured meats may be related to the development of COPD.
COPD as an autoimmune disease
There is mounting evidence that there may be an autoimmune component to COPD. Many individuals with COPD who have stopped smoking have active inflammation in the lungs. The disease may continue to get worse for many years after stopping smoking due to this ongoing inflammation. This sustained inflammation is thought to be mediated by autoantibodies and autoreactive T cells.[18][19][20]
Disease process
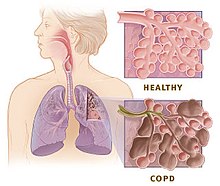
It is not fully understood how tobacco smoke and other inhaled particles damage the lungs to cause COPD. The most important processes causing lung damage are:
- Oxidative stress produced by the high concentrations of free radicals in tobacco smoke.
- Cytokine release due to inflammation as the body responds to irritant particles such as tobacco smoke in the airway.
- Tobacco smoke and free radicals impair the activity of antiprotease enzymes such as alpha 1-antitrypsin, allowing protease enzymes to damage the lung.
Pathology
Chronic bronchitis
Lung damage and inflammation in the large airways results in chronic bronchitis. Chronic bronchitis is defined in clinical terms as a cough with sputum production on most days for 3 months of a year, for 2 consecutive years[21]. In the airways of the lung, the hallmark of chronic bronchitris is an increased number (hyperplasia) and increased size (hypertrophy) of the goblet cells and mucous glands of the airway. As a result, there is more mucus than usual in the airways, contributing to narrowing of the airways and causing a cough with sputum. Microscopically there is infiltration of the airway walls with inflammatory cells. Inflammation is followed by scarring and remodeling that thickens the walls and also results in narrowing of the airways. As chronic bronchitis progresses, there is squamous metaplasia (an abnormal change in the tissue lining the inside of the airway) and fibrosis (further thickening and scarring of the airway wall). The consequence of these changes is a limitation of airflow[22]. Patients suffering from COPD that present primarily chronic bronchitis rather than emphysema are commonly referred to as "blue bloaters" because of the bluish color of the skin and lips (cyanosis) seen in them.
Emphysema

Lung damage and inflammation of the air sacs (alveoli) results in emphysema. Emphysema is defined as enlargement of the air spaces distal to the terminal bronchioles, with destruction of their walls.[21] The destruction of air space walls reduces the surface area available for the exchange of oxygen and carbon dioxide during breathing. It also reduces the elasticity of the lung itself, which results in a loss of support for the airways that are embedded in the lung. These airways are more likely to collapse causing further limitation to airflow. The effort made by patients suffering from emphysema during exhalation, causes a pink color in their faces, hence the term commonly used to refer to them, "pink puffers".
Pathophysiology
Narrowing of the airways reduces the rate at which air can flow to and from the air sacs (alveoli) and limits the effectiveness of the lungs. In COPD, the greatest reduction in air flow occurs when breathing out (during expiration) because the pressure in the chest tends to compress rather than expand the airways. In theory, air flow could be increased by breathing more forcefully, increasing the pressure in the chest during expiration. In COPD, there is often a limit to how much this can actually increase air flow, a situation known as expiratory flow limitation[23].
If the rate of airflow is too low, a person with COPD may not be able to completely finish breathing out (expiration) before he or she needs to take another breath. This is particularly common during exercise when breathing has to be faster. A little of the air of the previous breath remains within the lungs when the next breath is started. When this happens, there is an increase in the volume of air in the lungs, a process called dynamic hyperinflation.[23]
Dynamic hyperinflation is closely linked to shortness of breath (dyspnea) in COPD[24]. It is less comfortable to breathe with hyperinflation because it takes more effort to move the lungs and chest wall when they are already stretched by hyperinflation.
Another factor contributing to shortness of breath in COPD is the loss of the surface area available for the exchange of oxygen and carbon dioxide with emphysema. This reduces the rate of transfer of these gasses between the body and the atmosphere and can lead to low oxygen and high carbon dioxide levels in the body. A person with emphysema may have to breathe faster or more deeply to compensate, which can be difficult to do if there is also flow limitation or hyperinflation.
Some people with advanced COPD do manage to breathe fast to compensate, but usually have dyspnea as a result. Others, who may be less short of breath, tolerate low oxygen and high carbon dioxide levels in their bodies but this can eventually lead to headaches, drowsiness and heart failure.
Advanced COPD can lead to complications beyond the lungs such as weight loss (cachexia), pulmonary hypertension and heart failure (cor pulmonale). Osteoporosis, heart disease, muscle wasting and depression are all more common in people with COPD[1].
Acute exacerbations of COPD
An acute exacerbation of COPD is a sudden worsening of COPD symptoms (shortness of breath, quantity and color of phlegm) that typically lasts for several days. It may be triggered by an infection with bacteria or viruses or by environmental pollutants. Typically, infections cause 75% or more of the exacerbations; bacteria can roughly be found in 25% of cases, viruses in another 25%, and both viruses and bacteria in another 25%. Airway inflammation is increased during the exacerbation resulting in increased hyperinflation, reduced expiratory air flow and worsening of gas transfer.[1]
Diagnosis
The diagnosis of COPD should be considered in anyone who has dyspnea, chronic cough or sputum production, and/or a history of exposure to risk factors for the disease such as regular tobacco smoking[1][25]. No single symptom or sign can adequately confirm or exclude the diagnosis of COPD[26] although COPD is uncommon under the age of 40 years.
Spirometry
The diagnosis of COPD is confirmed by spirometry[1], a test that measures breathing. Spirometry measures the forced expiratory volume in one second (FEV1) which is the greatest volume of air that can be breathed out in the first second of a large breath. Spirometry also measures the forced vital capacity (FVC) which is the greatest volume of air that can be breathed out in a whole large breath. Normally at least 70% of the FVC comes out in the first second (i.e. the FEV1/FVC ratio is >70%). In COPD, this ratio is less than normal, (i.e. FEV1/FVC ratio is <70%) even after a bronchodilator medication has been given.
Spirometry can help to determine the severity of COPD.[1] The FEV1 (measured post-bronchodilator) is expressed as a percent of a predicted "normal" value based on a person's age, gender, height and weight:
| Severity of COPD | FEV1 % predicted |
|---|---|
| Mild | ≥80 |
| Moderate | 50-79 |
| Severe | 30-49 |
| Very severe | <30 or Chronic respiratory failure symptoms |
The severity of COPD also depends on the severity of dyspnea and exercise limitation. These and other factors can be combined with spirometry results to obtain a COPD severity score that takes multiple dimensions of the disease into account[27].
Other tests
An x-ray of the chest may show an over-expanded lung (hyperinflation) and can be useful to help exclude other lung diseases. Complete pulmonary function tests with measurements of lung volumes and gas transfer may also show hyperinflation and can discriminate between COPD with emphysema and COPD without emphysema. A high-resolution computed tomography scan of the chest may show the distribution of emphysema throughout the lungs and can also be useful to exclude other lung diseases.
A blood sample taken from an artery can be tested for blood gas levels which may show low oxygen levels (hypoxemia) and/or high carbon dioxide levels (respiratory acidosis). A blood sample taken from a vein may show a high blood count (reactive polythycaemia), a reaction to long-term hypoxemia.
Management
There is currently no cure for COPD; however, COPD is both a preventable and treatable disease.[1] Clinical practice guidelines for the management of COPD are available from the Global Initiative for Chronic Obstructive Lung Disease (GOLD),[28] a collaboration that includes the World Health Organization and the U.S. National Heart, Lung, and Blood Institute. For COPD exacerbations a meta analysis found that systemic corticosteroids were effective for all patients and that antibiotics and noninvasive positive pressure ventilation (NPPV) were effective for inpatients.[29]
Smoking cessation
Smoking cessation is one of the most important factors in slowing down the progression of COPD. Once COPD has been diagnosed, stopping smoking slows down the rate of progression of the disease. Even at a late stage of the disease it can significantly reduce the rate of deterioration in lung function and delay the onset of disability and death.[22] It is the only standard intervention that can improve the rate of progression of smoking-related COPD.
Smoking cessation starts with an individual decision to stop smoking that leads to an attempt at quitting. Often several attempts are required before long-term smoking cessation is achieved[30]. Some smokers can achieve long-term smoking cessation through "willpower" alone. However smoking is highly addictive and many smokers need further support to quit. The chance of successfully stopping smoking can be greatly improved through social support, engagement in a smoking cessation programme and the use of drugs such as nicotine replacement therapy, bupropion and varenicline.[30]
The policies of governments, public health agencies and anti-smoking organizations can reduce smoking rates by encouraging smoking cessation and discouraging people from starting smoking.[30] These policies are important strategies in the prevention of COPD.
Occupational health
Measures can be taken to reduce the likelihood that workers in at-risk industries such as coal mining will develop COPD. Some examples of these measures are: education of workers and management about the risks, promoting smoking cessation, surveillance of workers for early signs of COPD, the use of personal dust monitors, the use of respirators and dust control[31]. Dust control can be achieved by improving ventilation, using water sprays and by using mining techniques that minimize dust generation. If a worker develops COPD, further lung damage can be reduced by avoiding ongoing dust exposure, for example by changing the work role.
Environmental Change
Due to the individual differences in the causes and susceptibility to COPD, changes in the local environment of a COPD sufferer may have a significant and often subjectively subtle influence in the long term prognosis for the disease. Potential lung irritants such as common house dust, as well as such things as dust mites in bedding, (together with the rate at which bedding is changed/washed) as well as such domestic variables as carpets versus wooden floors, general or specific household dusts, cooking fumes etc, are all potential exacerbating influences in the progression of the disease. Sufferers are therefore strongly advised to discuss such factors with their health care provider.
Pharmacotherapy
Bronchodilators
There are several types of bronchodilators used clinically with varying efficacy: β2 agonists, M3 antimuscarinics, leukotriene antagonists, cromones and xanthines.[32] These drugs relax the smooth muscles of the airway allowing for improved airflow. The change in FEV1 may not be substantial, but changes in the vital capacity are significant. Many patients feel less breathless after taking bronchodilators.
β2 agonists
There are several highly specific β2 agonists available. Salbutamol (Ventolin) is the most widely used short acting β2 agonist to provide rapid relief and should be prescribed as a front line therapy for all classes of patients. Other β2 agonists are Bambuterol, Clenbuterol, Fenoterol, and Formoterol. Long acting β2 agonists (LABAs) such as Salmeterol act too slowly to be used as relief for dypsnea so these drugs should be used as maintenance therapy in the appropriate patient population. The TORCH study showed that LABA therapy reduced COPD exacerbation frequency over a 3 year period, compared to placebo.[33] An increased risk is associated with long acting β2 agonists due to decreased sensitivity to inflammation so generally the use of a concomitant corticosteroid is indicated[34][35][36].
M3 muscarinic antagonists (anticholinergics)
M3 muscarinic antagonists are anticholinergics. Specific antimuscarinics were found to provide effective relief to COPD. Inhaled antimuscarinics have the advantage of avoiding endocrine and exocrine M3 receptors. The quaternary M3 muscarinic antagonist Ipratropium is widely prescribed with the β2 agonist salbutamol. [1]. Ipratropium formerly was offered combined with salbutamol (Combivent) and with fenoterol (Duovent) but due to the CFC propellant, these products have been withdrawn.
Tiotropium provides improved specificity for M3 muscarinic receptors. It is a long acting muscarinic antagonist that has shown good efficacy in the reduction of exacerbations of COPD, especially when combined with a LABA and inhaled steroid.[37]
Cromones
Cromones are mast cell stabilizers that are thought to act on a chloride channel found on mast cells that help reduce the production of histamine and other inflammatory factors. Chromones are also thought to act on IgE-regulated calcium channels on mast cells. Cromoglicate and Nedocromil, which has a longer half-life, are two chromones available.[38]
Leukotriene antagonists
More recently leukotriene antagonists block the signalling molecules used by the immune system. Montelukast, Pranlukast, Zafirlukast are some of the leukotrienes antagonists.[39]These agents have not been tested in good, controlled trials, [40] and as such, there is no data to support the use of these agents in COPD.[41]
Xanthines
Theophylline is the prototype of the xanthine class of drug. Teas are natural sources of methylxanthines, xanthines and caffeine while cocoa is a natural source of theobromine. Caffeine is approximately 16% metabolized into theophylline. Nebulized theophylline is used in the EMR for treatment of dyspnea (Difficulty in breathing). Patients need continual monitoring as theophylline has a narrow therapeutic range. More aggressive EMR interventions include IV H1 antihistamines and IM dexamethasone.
Theophylline antagonizes phosphodiesterase, and small reductions in COPD exacerbation rates have been demonstrated.[42] The investigative phosphodiesterase-4 antagonists, roflumilast and cilomilast have completed Phase-2 clinical trials.
Corticosteroids
Enteral and parenteral corticosteroid therapy has long been the mainstay of treatment of COPD, and is known to reduce length of stay in hospital. Similarly, inhaled corticosteroids (specifically glucocorticoids) act in the inflammatory cascade and improve airway function considerably,[22] and have been shown in the ISOLDE trial to reduce the number of COPD exacerbations by 25%.[43] Corticosteroids are often combined with bronchodilators in a single inhaler. Some of the more common inhaled steroids in use are beclomethasone, mometasone, and fluticasone.
Salmeterol and fluticasone are combined (Advair), however the reduction in death from all causes among patients with COPD in the combination therapy group did not reach the predetermined level of statistical significance.[44][45]
TNF antagonists
Tumor necrosis factor antagonists (TNF) are the most recent class of medications designed to deal with refractory cases. Tumor necrosis factor-alpha is a cachexin or cachectin and is considered a so-called biological drug. They are considered immunosopressive with attendant risks. These rather expensive drugs include infliximab, adalimumab and etanercept.[46]Infliximab has been trialled in COPD with no evidence of benefit, with the possibility of harm. This was a relatively small study (77-79 patients in each arm).[47]
Supplemental oxygen

In general, long-term oxygen therapy is reserved for individuals with COPD who have arterial hypoxemia (PaO2 less than 55 mm Hg), or a PaO2 between 55 and 60 mm Hg with evidence of pulmonary hypertension, cor pulmonale, or secondary erythrocytosis (hematocrit >55%). In these patients, continuous home oxygen therapy (for >15 h/d) sufficient to correct hypoxemia has been shown to improve survival. [48] The use of low flow oxygen may be necessary in some patients because in the COPD patient, control of respiration is driven mainly by the blood oxygen level rather than the carbon dioxide level, increased oxygen delivery can diminish this response and cause respiratory failure.
Vaccination
Patients with COPD should be routinely vaccinated against influenza (yearly), pneumococcus (every 5 years) and other diseases to prevent illness and reduce the possibility of death.[32]
Pulmonary rehabilitation
Pulmonary rehabilitation is a program of disease management, counseling and exercise coordinated to benefit the individual.[49] Pulmonary rehabilitation has been shown to relieve difficulties breathing and fatigue. It has also been shown to improve the sense of control a patient has over their disease as well as their emotions.[50]
Diet
A recent French study conducted over 12 years with almost 43,000 men concluded that eating a Mediterranean diet "halves the risk of serious lung disease like emphysema and bronchitis". [51][52]
Treatment
Treatment for COPD includes inhalers that dilate the airways (bronchodilators) and sometimes theophylline. The COPD patient must stop smoking. In some cases inhaled steroids are used to suppress lung inflammation and, in severe cases or flare-ups, intravenous or oral steroids are given.
Antibiotics are used during flare-ups of symptoms as infections can cause acute exacerbations of COPD, though they have been shown to have only a small effect.[53] Chronic, low-flow oxygen, non-invasive ventilation, or intubation may be needed in some cases. Surgery to remove parts of the disease lung has been shown to be helpful for some patients with COPD.[要出典]
Lung rehabilitation programs may help some patients.
Lung transplant is sometimes performed for severe cases (preferentially for younger sufferers in many first world countries).
Support groups
The stress of illness can often be helped by joining a support group where members share common experiences and problems. [7]
Prognosis
A good prognosis of COPD relies on an early diagnosis and prompt treatment. Most patients will have improvement in lung function once treatment is started, however eventually signs and symptoms will worsen as COPD progresses. The median survival is about 10 years if two-thirds of expected lung function was lost by diagnosis.
"End Stages" of COPD are diagnosed as the normal ratio of carbon dioxide being inordinately higher than the volume of oxygen in the bloodstream. Although end stage COPD may mean death is imminent in cases dealing with degenerative diseases, forced oxygen therapy and other treatments may successfully be used to prolong life but have not been proven to be successful for the long run. These types of therapy may be successfully used in COPD cases caused by curable diseases such as bronchitis.
Bronchitis
Acute bronchitis usually resolves in 2-10 years.
Emphysema
The outcome is better for patients with less damage to the lungs who stop smoking any substance immediately (a fact that cannot be over emphasised). Still, patients with extensive lung damage may live for many years, so predicting prognosis is very difficult. Death may occur from respiratory failure, pneumonia, or other complications, such as influenza.
Pneumoconiosis
The outcome is good for patients with minimal damage to the lung. However, patients with extensive lung damage may live for many years so predicting prognosis is difficult. Death may occur from respiratory failure, pneumonia, cor pulmonale or other complications.
Pulmonary neoplasms
The stage of the tumor(s) has a major impact on neoplasm prognosis. Staging is the process of determining tumor size, growth rate, potential metastasis, lymph node involvement, treatment options and prognosis. Two-year prognosis for limited small cell pulmonary neoplasms is twenty percent and for extensive disease five percent. The average life expectancy for someone with recurrent small cell pulmonary neoplasms is two to three months.[54]
The 5-year overall survival rate for pulmonary neoplasms is 14%.[55]
Epidemiology
According to the World Health Organization (WHO), 80 million people suffer from moderate to severe COPD and 3 million died due to it in 2005. The WHO predicts that by 2030, it will be the 3rd largest cause of mortality worldwide.[56]
Since COPD is not diagnosed until it becomes clinically apparent, prevalence and mortality data greatly underestimate the socioeconomic burden of COPD.[32] In the UK, COPD accounts for about 7% of all days of sickness related absence from work.[22]
Smoking rates in the industrialized world have continued to fall, causing rates of emphysema and pulmonary neoplasms to slowly decline.
Footnotes
- ^ a b c d e f g h i Rabe KF, Hurd S, Anzueto A, et al (2007). “Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary”. Am. J. Respir. Crit. Care Med. 176 (6): 532–55. doi:10.1164/rccm.200703-456SO. PMID 17507545.
- ^ Hogg JC, Chu F, Utokaparch S, et al (2004). “The Nature of Small-Airway Obstruction in Chronic Obstructive Pulmonary Disease”. New England Journal of Medicine 350 (26): 2645–53. doi:10.1056/NEJMoa032158. PMID 15215480.
- ^ COPD (Chronic Obstructive Pulmonary Disease)
- ^ “2007 NHLBI Morbidity and Mortality Chart Book”. 2008年6月6日閲覧。
- ^ Mahler DA (2006). “Mechanisms and measurement of dyspnea in chronic obstructive pulmonary disease”. Proceedings of the American Thoracic Society 3 (3): 234–8. doi:10.1513/pats.200509-103SF. PMID 16636091.
- ^ U.S. National Heart Lung and Blood Institute - Signs and Symptoms
- ^ a b MedlinePlus Medical Encyclopedia: Chronic obstructive pulmonary disease
- ^ MedicineNet.com - COPD signs & symptoms
- ^ MedicineNet.com - COPD causes
- ^ Løkke A, Lange P, Scharling H, Fabricius P, Vestbo J (2006). “Developing COPD: a 25 year follow up study of the general population”. Thorax 61 (11): 935–9. doi:10.1136/thx.2006.062802. PMID 17071833.
- ^ Devereux, Graham (May 2006). “ABC of chronic obstructive pulmonary disease. Definition, epidemiology, and risk factors”. BMJ 332 (7550): 1142–4. doi:10.1136/bmj.332.7550.1142. PMC 1459603. PMID 16690673.
- ^ Hnizdo E, Vallyathan V (April 2003). “Chronic obstructive pulmonary disease due to occupational exposure to silica dust: a review of epidemiological and pathological evidence”. Occup Environ Med 60 (4): 237–43. PMC 1740506. PMID 12660371.
- ^ a b Loscalzo, Joseph; Fauci, Anthony S.; Braunwald, Eugene; Dennis L. Kasper; Hauser, Stephen L; Longo, Dan L. (2008). Harrison's Principles of Internal Medicine (17th Edition ed.). McGraw-Hill Professional. ISBN 0-07-146633-9
- ^ Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM (September 2006). “Global burden of COPD: systematic review and meta-analysis”. Eur. Respir. J. 28 (3): 523–32. doi:10.1183/09031936.06.00124605. PMID 16611654.
- ^ Kennedy SM, Chambers R, Du W, Dimich-Ward H (December 2007). “Environmental and occupational exposures: do they affect chronic obstructive pulmonary disease differently in women and men?”. Proceedings of the American Thoracic Society 4 (8): 692–4. doi:10.1513/pats.200707-094SD. PMID 18073405.
- ^ Silverman EK, Chapman HA, Drazen JM, et al (June 1998). “Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis”. Am. J. Respir. Crit. Care Med. 157 (6 Pt 1): 1770–8. PMID 9620904.
- ^ MedlinePlus Encyclopedia 000091
- ^ Feghali-Bostwick CA, Gadgil AS, Otterbein LE, et al (January 2008). “Autoantibodies in patients with chronic obstructive pulmonary disease”. Am. J. Respir. Crit. Care Med. 177 (2): 156–63. doi:10.1164/rccm.200701-014OC. PMID 17975205.
- ^ Lee SH, Goswami S, Grudo A, et al (May 2007). “Antielastin autoimmunity in tobacco smoking-induced emphysema”. Nat. Med. 13 (5): 567–9. doi:10.1038/nm1583. PMID 17450149.
- ^ Rutgers, Steven R.; Postma, Dirkje S.; Ten Hacken, Nick H. .T.; Kauffman, Henk F.;van der Mark,Thomas W; Koeter, Gerard H.; Timens, Wim (2000). “Ongoing airway inflammation in patients with COPD who do not currently smoke”. Thorax 55 (1): 12–18. doi:10.1136/thorax.55.1.12. PMC PMC1745599. PMID 10607796.
- ^ a b Longmore, J. M.; Murray Longmore; Wilkinson, Ian; Supraj R. Rajagopalan (2004). Oxford handbook of clinical medicine. Oxford [Oxfordshire]: Oxford University Press. pp. 188-189. ISBN 0-19-852558-3
- ^ a b c d Kumar P, Clark M (2005). Clinical Medicine, 6ed. Elsevier Saunders. pp 900-901. ISBN 0702027634.
- ^ a b Calverley PM, Koulouris NG (2005). “Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology”. Eur Respir J 25: 186-199. PMID 15640341.
- ^ O'Donnell DE (2006). “Hyperinflation, Dyspnea, and Exercise Intolerance in Chronic Obstructive Pulmonary Disease”. The Proceedings of the American Thoracic Society 3: 180-184. PMID 16565429.
- ^ Badgett RG, Tanaka DJ, Hunt DK, et al (1994). “The clinical evaluation for diagnosing obstructive airways disease in high-risk patients”. Chest 106 (5): 1427–31. doi:10.1378/chest.106.5.1427. PMID 7956395.
- ^ Holleman DR, Simel DL (1995). “Does the clinical examination predict airflow limitation?”. JAMA 273 (4): 313–9. doi:10.1001/jama.273.4.313. PMID 7815660.
- ^ Celli BR, Cote CG, Marin JM, et al (March 2004). “The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease”. N. Engl. J. Med. 350 (10): 1005–12. doi:10.1056/NEJMoa021322. PMID 14999112.
- ^ Global Initiative for Chronic Obstructive Lung Disease.
- ^ Quon BS, Gan WQ, Sin DD (March 2008). “Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis”. Chest 133 (3): 756–66. doi:10.1378/chest.07-1207. PMID 18321904.
- ^ a b c “World Health Organization Tobacco Free Initiative - Policy recommendations for smoking cessation and treatment of tobacco dependence”. Template:Cite webの呼び出しエラー:引数 accessdate は必須です。
- ^ “National Institute of Occupational Safety and Health - Handbook for Dust Control in Mining”. Template:Cite webの呼び出しエラー:引数 accessdate は必須です。
- ^ a b c American Thoracic Society / European Respiratory Society Task Force (2005). Standards for the Diagnosis and Management of Patients with COPD. Version 1.2. New York: American Thoracic Society. http://www.thoracic.org/go/copd
- ^ Calverley PM, Anderson JA, Celli B, et al (2007). “Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease”. N. Engl. J. Med. 356 (8): 775–89. doi:10.1056/NEJMoa063070. PMID 17314337.
- ^ “MedWatch - Serevent Dear Healthcare Professional letter”. Template:Cite webの呼び出しエラー:引数 accessdate は必須です。
- ^ “GlaxoSmithKline - Press Archive GSK announces interim US Serevent study results”. Template:Cite webの呼び出しエラー:引数 accessdate は必須です。
- ^ “Medscape 527629 - please fix”. Template:Cite webの呼び出しエラー:引数 accessdate は必須です。
- ^ Aaron SD, Vandemheen KL, Fergusson D, et al (2007). “Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial”. Ann. Intern. Med. 146 (8): 545–55. PMID 17310045.
- ^ Howell JB, Altounyan RE (1967). “A double-blind trial of disodium cromoglycate in the treatment of allergic bronchial asthma”. Lancet 2 (7515): 539–42. PMID 4166895.
- ^ BROCKLEHURST WE (1960). “The release of histamine and formation of a slow-reacting substance (SRS-A) during anaphylactic shock”. J. Physiol. (Lond.) 151: 416–35. PMID 13804592.
- ^ From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2007. Available from: http://www.goldcopd.org.
- ^ Standards for the Diagnosis and Management of Patients with COPD, American Thoracic Society and the European Respiratory Society 2005. Available from: http://www.thoracic.org/sections/copd/resources/copddoc.pdf.
- ^ Zhou Y, Wang X, Zeng X, et al (2006). “Positive benefits of theophylline in a randomized, double-blind, parallel-group, placebo-controlled study of low-dose, slow-release theophylline in the treatment of COPD for 1 year”. Respirology 11 (5): 603–10. doi:10.1111/j.1440-1843.2006.00897.x. PMID 16916334.
- ^ Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK (2000). “Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial”. BMJ 320 (7245): 1297–303. doi:10.1136/bmj.320.7245.1297. PMID 10807619.
- ^ Calverley PM, Anderson JA, Celli B, et al (February 2007). “Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease”. N. Engl. J. Med. 356 (8): 775–89. doi:10.1056/NEJMoa063070. PMID 17314337.
- ^ Survival Of Subjects With Chronic Obstructive Pulmonary Disease (COPD) - Full Text View - ClinicalTrials.gov
- ^ Cell Signaling
- ^ Rennard SI, Fogarty C, Kelsen S, et al (May 2007). “The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease”. Am. J. Respir. Crit. Care Med. 175 (9): 926–34. doi:10.1164/rccm.200607-995OC. PMID 17290043.
- ^ “Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party”. Lancet 1 (8222): 681–6. (March 1981). PMID 6110912.
- ^ U.S. National Heart Lung and Blood Institute - Treatment
- ^ Lacasse Y, Brosseau L, Milne S, et al (2002). “Pulmonary rehabilitation for chronic obstructive pulmonary disease”. Cochrane database of systematic reviews (Online) (3): CD003793. PMID 12137716.
- ^ “BBC NEWS Health Med diet 'cuts lung disease risk'”. Template:Cite webの呼び出しエラー:引数 accessdate は必須です。
- ^ Aniwidyaningsih W, Varraso R, Cano N, Pison C (July 2008). “Impact of nutritional status on body functioning in chronic obstructive pulmonary disease and how to intervene”. Curr Opin Clin Nutr Metab Care 11 (4): 435–442. doi:10.1097/MCO.0b013e3283023d37. PMID 18542004.
- ^ Gibson, et al. Evidence-based Respiratory Medicine. Blackwell Publishing, 2005. ISBN 072791605X. pp. 390-392.
- ^ “Site Map: Lung.com”. Template:Cite webの呼び出しエラー:引数 accessdate は必須です。
- ^ Jameson, J. N. St C.; Dennis L. Kasper; Harrison, Tinsley Randolph; Braunwald, Eugene; Fauci, Anthony S.; Hauser, Stephen L; Longo, Dan L. (2005). “John D. Minna, Neoplasms of the Lung”. Harrison's principles of internal medicine. New York: McGraw-Hill Medical Publishing Division. pp. 506. ISBN 0-07-140235-7
- ^ WHO - COPD
External links
- National Heart, Lung and Blood Institute - COPD U.S. NHLBI Information for Patients and the Public page.
- Chronic Respiratory Disease - leading research and articles on respiratory disease
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)
- Chronic Obstructive Pulmonary Disease - medical information and informative articles on COPD
