ラディキシン
表示
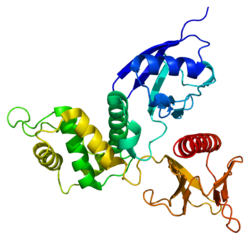
ラディキシン(Radixin)は、ヒトではRDX遺伝子によりコードされるタンパク質である[5][6][7]。モエシン、エズリンとともにERMファミリーを構成する。
ラディキシンは細胞骨格タンパク質で、アクチンと細胞膜の結合に重要な役割を果たしている可能性がある。エズリンやモエシンと高度の配列類似性がみられる。RDX遺伝子は11q23に位置することが蛍光 in situ ハイブリダイゼーションにより特定されている。不完全なものをコードする偽遺伝子(RDXP2)がXp21.3に、イントロンを欠く別の偽遺伝子(RDXP1)が11pに存在する[7]。
相互作用
[編集]ラディキシンは、GNA13とタンパク質間相互作用する[8]。
出典
[編集]- ^ a b c GRCh38: Ensembl release 89: ENSG00000137710 - Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000032050 - Ensembl, May 2017
- ^ Human PubMed Reference:
- ^ Mouse PubMed Reference:
- ^ “Molecular cloning, cDNA sequence, and chromosomal assignment of the human radixin gene and two dispersed pseudogenes”. Genomics 16 (1): 199-206. (June 1993). doi:10.1006/geno.1993.1159. PMID 8486357.
- ^ “Mutations of the RDX gene cause nonsyndromic hearing loss at the DFNB24 locus”. Hum Mutat 28 (5): 417-23. (April 2007). doi:10.1002/humu.20469. PMID 17226784.
- ^ a b “Entrez Gene: RDX radixin”. 2020年3月31日閲覧。
- ^ “Conformational activation of radixin by G13 protein alpha subunit”. J. Biol. Chem. 275 (34): 26206-12. (August 2000). doi:10.1074/jbc.M001863200. PMID 10816569.
関連文献
[編集]- “Radixin: cytoskeletal adopter and signaling protein”. Int. J. Biochem. Cell Biol. 36 (11): 2131-6. (2005). doi:10.1016/j.biocel.2003.11.018. PMID 15313460.
- “Human immunodeficiency virus (HIV)-1 proteins and cytoskeleton: partners in viral life and host cell death”. Cell Death Differ. 12 Suppl 1: 932-41. (2006). doi:10.1038/sj.cdd.4401582. PMID 15818415.
- “A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites”. J. Cell Sci. 103 (1): 131-43. (1992). PMID 1429901.
- “Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway”. J. Cell Biol. 135 (1): 37-51. (1996). doi:10.1083/jcb.135.1.37. PMC 2121020. PMID 8858161.
- “Normalization and subtraction: two approaches to facilitate gene discovery”. Genome Res. 6 (9): 791-806. (1997). doi:10.1101/gr.6.9.791. PMID 8889548.
- “Expression of NF2-encoded merlin and related ERM family proteins in the human central nervous system”. J. Neuropathol. Exp. Neurol. 56 (6): 735-42. (1997). doi:10.1097/00005072-199756060-00011. PMID 9184664.
- “Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein”. J. Biol. Chem. 272 (37): 23371-5. (1997). doi:10.1074/jbc.272.37.23371. PMID 9287351.
- “ERM (Ezrin/Radixin/Moesin)-based Molecular Mechanism of Microvillar Breakdown at an Early Stage of Apoptosis”. J. Cell Biol. 139 (3): 749-58. (1997). doi:10.1083/jcb.139.3.749. PMC 2141718. PMID 9348291.
- “NHE-RF, a regulatory cofactor for Na(+)-H+ exchange, is a common interactor for merlin and ERM (MERM) proteins”. J. Biol. Chem. 273 (3): 1273-6. (1998). doi:10.1074/jbc.273.3.1273. PMID 9430655.
- “Rho-Kinase Phosphorylates COOH-terminal Threonines of Ezrin/Radixin/Moesin (ERM) Proteins and Regulates Their Head-to-Tail Association”. J. Cell Biol. 140 (3): 647-57. (1998). doi:10.1083/jcb.140.3.647. PMC 2140160. PMID 9456324.
- “Ezrin/Radixin/Moesin (ERM) Proteins Bind to a Positively Charged Amino Acid Cluster in the Juxta-Membrane Cytoplasmic Domain of CD44, CD43, and ICAM-2”. J. Cell Biol. 140 (4): 885-95. (1998). doi:10.1083/jcb.140.4.885. PMC 2141743. PMID 9472040.
- “Mapping of ezrin dimerization using yeast two-hybrid screening”. Biochem. Biophys. Res. Commun. 243 (3): 874-7. (1998). doi:10.1006/bbrc.1998.8196. PMID 9501018.
- “Interaction of radixin with Rho small G protein GDP/GTP exchange protein Dbl”. Oncogene 16 (25): 3279-84. (1998). doi:10.1038/sj.onc.1201874. PMID 9681826.
- “The TSC1 tumour suppressor hamartin regulates cell adhesion through ERM proteins and the GTPase Rho”. Nat. Cell Biol. 2 (5): 281-7. (2000). doi:10.1038/35010550. PMID 10806479.
- “Conformational activation of radixin by G13 protein alpha subunit”. J. Biol. Chem. 275 (34): 26206-12. (2000). doi:10.1074/jbc.M001863200. PMID 10816569.
- “Crystallographic characterization of the radixin FERM domain bound to the cytoplasmic tail of the adhesion protein ICAM-2”. Acta Crystallogr. D 57 (Pt 6): 891-2. (2001). doi:10.1107/S0907444901005716. PMID 11375520.
- “Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes”. Nat. Genet. 31 (3): 320-5. (2002). doi:10.1038/ng905. PMID 12068294.
- “Functional binding interaction identified between the axonal CAM L1 and members of the ERM family”. J. Cell Biol. 157 (7): 1105-12. (2002). doi:10.1083/jcb.200111076. PMC 2173555. PMID 12070130.
- “The TSC1 tumor suppressor hamartin interacts with neurofilament-L and possibly functions as a novel integrator of the neuronal cytoskeleton”. J. Biol. Chem. 277 (46): 44180-6. (2003). doi:10.1074/jbc.M207211200. PMID 12226091.