「遺伝性非ポリポーシス大腸癌」の版間の差分
←新しいページ: '{{Infobox_Disease | Name = {{PAGENAME}} | Image = | Caption = | DiseasesDB = 5812 | ICD10 = {{ICD10|C|18||c|15}}-...' |
(相違点なし)
|
2010年8月1日 (日) 02:59時点における版
| 遺伝性非ポリポーシス大腸癌 | |
|---|---|
| 概要 | |
| 診療科 | 腫瘍学 |
| 分類および外部参照情報 | |
| ICD-10 | C18-C20 |
| ICD-9-CM | 153.0-154.1 |
| OMIM | 120435 609310 114400 |
| DiseasesDB | 5812 |
| MeSH | D003123 |
遺伝性非ポリポーシス大腸癌 (いでんせい・ひポリポーシス・だいちょうがん、英:Hereditary nonpolyposis colorectal cancer; HNPCC )とは常染色体優性の遺伝的素因による大腸癌である。[1] 子宮癌、卵巣癌、胃癌、小腸腫瘍、胆嚢癌、尿管癌、脳腫瘍、皮膚癌など、他臓器における発癌もしばしばみられる。これらの発癌リスクの上昇はDNAミスマッチ修復酵素の変異による。
定義
ヘンリー・リンチ(クレイトン大学医学部教授),[2] が1966年に報告した。[3] 当初彼は「家族内集積発癌症候群」と記載していた。 「リンチ症候群」とは他の執筆者によって名付けられ、リンチ自身は1985年にHNPCCという概念を提唱した。それ以降これらの二つはそれぞれ用いられていたが、近年の分子生物学の進歩によりHNPCCの本質が解明された。[4] 合併症については別に「ベセスダ基準」が制定されている。[5][6][7]
分類
マイクロサテライト不安定性[8]により病理組織学的には3つのグループに大きく分類される。
- マーカーで30%以上の不安定性を示す高不安定性(MSI-high)
- 不安定性30%未満を示す低不安定性(MSI-low)
- 不安定性を全く示さない安定性(MSI-stable)
徴候と症状
大腸癌の危険性
HNPCCの遺伝的素因を持つヒトの一生を通じての大腸癌発癌の可能性は80%である。これらの癌の3分の2は右半結腸にみられる。アムステルダム基準に合致した家系での大腸癌の発症平均年齢は44歳であった。eria. Also, women with HNPCC have a 30-50% lifetime risk of endometrial cancer. The average age of diagnosis of endometrial cancer is about 46 years. Among women with HNPCC who have both colon and endometrial cancer, about half present first with endometrial cancer. In HNPCC, the mean age of diagnosis of gastric cancer is 56 years of age with intestinal-type adenocarcinoma being the most commonly reported pathology. HNPCC-associated ovarian cancers have an average age of diagnosis of 42.5 years-old; approximately 30% are diagnosed before age 40 years. Other HNPCC-related cancers have been reported with specific features: the urinary tract cancers are transitional carcinoma of the ureter and renal pelvis; small bowel cancers occur most commonly in the duodenum and jejunum; the central nervous system tumor most often seen is glioblastoma.
遺伝学
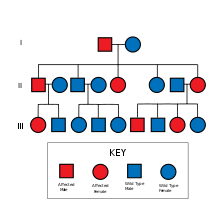
HNPCCはDNAミスマッチ修復能を欠損しており、マイクロサテライト不安定性(MSI)を呈する。高不安定性を示すことはHNPCCの特徴である。マイクロサテライト不安定性は癌組織でも検出される。[9]
HNPCC is known to be associated with mutations in genes involved in the DNA mismatch repair pathway
| OMIM name | Genes implicated in HNPCC | Frequency of mutations in HNPCC families | Locus | First publication |
|---|---|---|---|---|
| HNPCC1 (120435) | MSH2 | approximately 60% | 2p22 | Fishel et al., 1993[10] |
| HNPCC2 (609310) | MLH1 | approximately 30% | 3p21 | Papadopoulos et al., 1994[11] |
| HNPCC5 | MSH6 | 7-10% | 2p16 | |
| HNPCC4 | PMS2 | relatively infrequent[12], <5% [要出典] | 7p22 | |
| HNPCC3 | PMS1 | case report[13] | 2q31-q33 | |
| HNPCC6 | TGFBR2 | case report[14] | 3p22 | |
| HNPCC7 | MLH3 | disputed[15] | 14q24.3 |
Patients with MSH6 mutations are more likely to be Amsterdam criteria II-negative.[16] The presentation with MSH6 is slightly different than with MLH1 and MSH2, and the term "MSH6 syndrome" has been used to describe this condition.[17] In one study, the Bethesda guidelines were more sensitive than the Amsterdam Criteria in detecting it.[18]
Up to 39% of families with mutations in an HNPCC gene do not meet the Amsterdam criteria.[要出典] Therefore, families found to have a deleterious mutation in an HNPCC gene should be considered to have HNPCC regardless of the extent of the family history. This also means that the Amsterdam criteria fail to identify many patients at risk for Lynch syndrome. Improving the criteria for screening is an active area of research, as detailed in the Screening Strategies section of this article.
HNPCC is inherited in an autosomal dominant manner. Most people with HNPCC inherit the condition from a parent. However, due to incomplete penetrance, variable age of cancer diagnosis, cancer risk reduction, or early death, not all patients with an HNPCC gene mutation have a parent who had cancer. Some patients develop HNPCC de-novo in a new generation, without inheriting the gene. These patients are often only identified after developing an early-life colon cancer. Parents with HNPCC have a 50% chance to pass the gene on to each child.
スクリーニング検査
DNAミスマッチ修復遺伝子の検査はHNPCC家系では勧められる。日本においては健康保険適応となっている。 癌を発症した場合には癌組織と正常組織でのマイクロサテライト不安定性を調べることができるし、組織採取を行わない場合には採血により白血球での検査を行う。
アムステルダム基準
The following are the Amsterdam criteria in identifying high-risk candidates for molecular genetic testing:[19]
アムステルダム基準:
- 3人以上が家族内で大腸癌の確定診断を得ており、その内のひとりは他のふたりの一度近親者である。
- 連続した2世代で罹患している。
- ひとり以上の50歳未満での大腸癌患者がいる。
- 家族性大腸ポリポーシスは除外されている。
アムステルダム基準II (新アムステルダム基準):
- Three or more family members with HNPCC-related cancers, one of whom is a first degree relative of the other two
- Two successive affected generations
- One or more of the HNPCC-related cancers diagnosed under age 50 years
- Familial adenomatous polyposis (FAP) has been excluded
診断
The Amsterdam clinical criteria identifies candidates for genetic testing, and genetic testing can make a diagnosis of Lynch syndrome. Genetic testing is commercially available and consists of a blood test.
管理
Surgery remains the front-line therapy for HNPCC. There is an ongoing controversy over the benefit of 5-fluorouracil-based adjuvant therapies for HNPCC-related colorectal tumours, particularly those in stages I and II.[20]
After reporting a null finding from their randomized controlled trial of aspirin (ASA) to prevent against the colorectal neoplasia of Lynch Syndrome,[21] Burn and colleagues have recently reported new data, representing a longer follow-up period than reported in the initial NEJM paper. These new data demonstrates a reduced incidence in Lynch Syndrome patients who were exposed to at least four years of high-dose aspirin, with a satisfactory risk profile.[22] These results have been widely covered in the media; future studies will look at modifying (lowering) the dose (to reduce risk associated with the high dosage of ASA. Individuals with Lynch Syndrome may wish to discuss the application of these results with their medical care team.
疫学
In the United States, about 160,000 new cases of colorectal cancer are diagnosed each year. Hereditary nonpolyposis colorectal cancer is responsible for approximately 2 percent to 7 percent of all diagnosed cases of colorectal cancer. The average age of diagnosis of cancer in patients with this syndrome is 44 years old, as compared to 64 years old in people without the syndrome. [23]
引用・参照
- ^ Kastrinos F, Mukherjee B, Tayob N, et al. (October 2009). “Risk of pancreatic cancer in families with Lynch syndrome”. JAMA 302 (16): 1790–5. doi:10.1001/jama.2009.1529. PMID 19861671.
- ^ School of Medicine :: Hereditary Cancer Center :: Creighton University
- ^ Lynch HT, Shaw MW, Magnuson CW, Larsen AL, Krush AJ (February 1966). “Hereditary factors in cancer. Study of two large midwestern kindreds”. Arch. Intern. Med. 117 (2): 206–12. doi:10.1001/archinte.117.2.206. PMID 5901552.
- ^ Bellizzi AM, Frankel WL (2009). “Colorectal cancer due to deficiency in DNA mismatch repair function: a review”. Advances in Anatomic Pathology 16 (6): 405–417. doi:10.1097/PAP.0b013e3181bb6bdc. PMID 19851131.
- ^ Gologan A, Krasinskas A, Hunt J, Thull DL, Farkas L, Sepulveda AR (November 2005). “Performance of the revised Bethesda guidelines for identification of colorectal carcinomas with a high level of microsatellite instability”. Arch. Pathol. Lab. Med. 129 (11): 1390–7. PMID 16253017.
- ^ Umar A, Boland CR, Terdiman JP, et al. (February 2004). “Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability”. J. Natl. Cancer Inst. 96 (4): 261–8. PMID 14970275.
- ^ Lipton LR, Johnson V, Cummings C, et al. (December 2004). “Refining the Amsterdam Criteria and Bethesda Guidelines: testing algorithms for the prediction of mismatch repair mutation status in the familial cancer clinic”. J. Clin. Oncol. 22 (24): 4934–43. doi:10.1200/JCO.2004.11.084. PMID 15611508.
- ^ http://jsft.umin.jp/hp/msikensa.html
- ^ Pathology of Hereditary Nonpolyposis Colorectal Cancer - JASS 910 (1): 62 - Annals of the New York Academy of Sciences
- ^ Fishel R, Lescoe M, Rao M, Copeland N, Jenkins N, Garber J, Kane M, Kolodner R (1993). “The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer”. Cell 75 (5): 1027–38. doi:10.1016/0092-8674(93)90546-3. PMID 8252616.
- ^ Papadopoulos N, Nicolaides N, Wei Y, Ruben S, Carter K, Rosen C, Haseltine W, Fleischmann R, Fraser C, Adams M (1994). “Mutation of a mutL homolog in hereditary colon cancer”. Science 263 (5153): 1625–9. doi:10.1126/science.8128251. PMID 8128251.
- ^ Thompson E, Meldrum CJ, Crooks R, et al. (March 2004). “Hereditary non-polyposis colorectal cancer and the role of hPMS2 and hEXO1 mutations”. Clin. Genet. 65 (3): 215–25. doi:10.1111/j.1399-0004.2004.00214.x. PMID 14756672.
- ^ Nicolaides NC, Papadopoulos N, Liu B, et al. (September 1994). “Mutations of two PMS homologues in hereditary nonpolyposis colon cancer”. Nature 371 (6492): 75–80. doi:10.1038/371075a0. PMID 8072530.
- ^ Lu SL, Kawabata M, Imamura T, et al. (May 1998). “HNPCC associated with germline mutation in the TGF-beta type II receptor gene”. Nat. Genet. 19 (1): 17–8. doi:10.1038/ng0598-17. PMID 9590282.
- ^ Ou J, Rasmussen M, Westers H, et al. (April 2009). “Biochemical characterization of MLH3 missense mutations does not reveal an apparent role of MLH3 in Lynch syndrome”. Genes Chromosomes Cancer 48 (4): 340–50. doi:10.1002/gcc.20644. PMID 19156873.
- ^ Ramsoekh D, Wagner A, van Leerdam ME, et al. (November 2008). “A high incidence of MSH6 mutations in Amsterdam criteria II-negative families tested in a diagnostic setting”. Gut 57 (11): 1539–44. doi:10.1136/gut.2008.156695. PMID 18625694.
- ^ Suchy J, Lubinski J (2008). “MSH6 syndrome”. Hered Cancer Clin Pract 6 (2): 103–104. doi:10.1186/1897-4287-6-2-103. PMC 2735474. PMID 19804606.
- ^ Goldberg Y, Porat RM, Kedar I, et al. (October 2009). “An Ashkenazi founder mutation in the MSH6 gene leading to HNPCC”. Fam. Cancer 9 (2): 141–50. doi:10.1007/s10689-009-9298-9. PMID 19851887.
- ^ Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999;116:1453-6. PMID 10348829.
- ^ Boland CR, Koi M, Chang DK, Carethers JM. The biochemical basis of microsatellite instability and abnormal immunohistochemistry and clinical behavior in Lynch Syndrome: from bench to bedside. Familial Cancer epub 2007; DOI 10.1007/s10689-007-9145-9
- ^ Burn J, Bishop DT, Mecklin JP, et al. (December 2008). “Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome”. N. Engl. J. Med. 359 (24): 2567–78. doi:10.1056/NEJMoa0801297. PMID 19073976.
- ^ “Aspirin Confers Long-Term Protective Effect in Lynch Syndrome Patients”. 2009年11月7日閲覧。
- ^ Cancer Information, Research, and Treatment for all Types of Cancer | OncoLink
