ククルビタン
| ククルビタン | |
|---|---|

| |
| 識別情報 | |
| CAS登録番号 | 65441-59-0 (5α/β) |
| ChEBI | |
| |
| 特性 | |
| 化学式 | C30H54 |
| モル質量 | 414.75 g mol−1 |
| 特記なき場合、データは常温 (25 °C)・常圧 (100 kPa) におけるものである。 | |
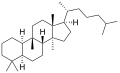
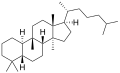
ククルビタン(Cucurbitane)は、化学式C30H54の化合物である。多環脂肪族炭化水素であり、特にトリテルペンに分類される。ラノスタンの異性体であり、メチル基が10位から9β位に移っている点が異なる[1][2]。
5αと5βの2つの立体異性体があり、これらは、5位の炭素原子における結合の掌性が異なる[1]。
-
5α-ククルビタン
-
5β-ククルビタン
誘導体[編集]
ククルビタン構造を基礎骨格として持つ化合物は、植物に多くみられ、そのうちのいくつかは重要なフィトメディシンである[3]。天然のククルビタン関連化合物には、以下のようなものがある。
- バルサミナペンタオール - Momordica balsamina由来[4]
- バルサミノールA - M. balsamina由来[4]
- バルサミノールB - M. balsamina由来[4]
- ブリジオシドA - Bryonia dioica由来[3]:64
- ブリオアマリド - B. dioica由来[3]:65,66
- チャランチン - ツルレイシ(Momordica charantia)[5]及びMomordica foetida[6]由来
- チャラントシド I-VIII - ツルレイシ由来[7]
- ククルバルサミノールB - M. balsamina由来[4]
- ククルバルサミノールA - M. balsamina由来[4]
- ククルビタシン A-L, O-T - M. balsamina由来[3][8] [9]:3–8
- ダチスコシド - Datisca glomerata由来[3]:16–19
- エンデカフィラシン A, B - Hemsleya endecaphyllaの根由来[9]:1,2
- ヘムスレシン A, B - H. endecaphyllaの根由来[9]
- レピドリド - 担子菌Russula lepida由来[10]
- カラビラゲニンE - M. balsamina由来[4]
- ケカダエンゴシド A, B, D, K - Trichosanthes tricuspidata由来[3]:57,58,67,68
- クグアシン A-S - ツルレイシの根由来[11][12]
- クグアグリコシド A-H - ツルレイシの茎と葉由来[13]
- モグロシド I-IV - ラカンカ(Siraitia grosvenorii)の果実由来[14]
- モモルジシン I, II, 28 - ツルレイシ由来[15][16]
- モモルジコシド A-S - ツルレイシの果実由来[7][17][18]
- ネオクグアグルコシド - ツルレイシの果実由来[19]
- ネオモグロシド - ラカンカの果実由来[20]
- ペンタノルククルビタシン A, B
- ペルセアピクロシド A - Persea mexicana由来[3]:44
- スカンデノシド R9 - Hemsleya panacis-scandens由来[3]:45
- スピノシド A, B[3]:61,62
関連項目[編集]
出典[編集]
- ^ a b IUPAC Commission on the Nomenclature of Organic Chemistry and IUPAC-IUB Commission on Biochemical Nomenclature (1969), The Nomenclature of Steroids — Revised Tentative Rules. European J. of Biochemistry, volume 10, 1-19
- ^ Satish Kumar and Raj Kumar (1991), Dictionary of Biochemistry. Anmol Publications, India
- ^ a b c d e f g h i Chen, Jian Chao; Chiu, Ming Hua; Nie, Rui Lin; Cordell, Geoffrey A.; Qiu, Samuel X. (2005). “Cucurbitacins and cucurbitane glycosides: structures and biological activities”. Natural Product Reports 22 (3): 386. doi:10.1039/b418841c. ISSN 0265-0568.
- ^ a b c d e f Ramalhete, Cátia; Mansoor, Tayyab A.; Mulhovo, Silva; Molnár, Joseph; Ferreira, Maria-José U. (2009). “Cucurbitane-Type Triterpenoids from the African PlantMomordica balsamina”. Journal of Natural Products 72 (11): 2009–2013. doi:10.1021/np900457u. ISSN 0163-3864.
- ^ M. M. Lolitkar and M. R. Rajarama Rao (1962), "Note on a Hypoglycaemic Principle Isolated from the fruits of Momordica charantia". Journal of the University of Bombay, volume 29, pages 223-224
- ^ “A neutral constituent of Momordica foetida”. Lloydia 38 (4): 361–2. (1975). PMID 1186439.
- ^ a b Akihisa, Toshihiro; Higo, Naoki; Tokuda, Harukuni; Ukiya, Motohiko; Akazawa, Hiroyuki; Tochigi, Yuichi; Kimura, Yumiko; Suzuki, Takashi et al. (2007). “Cucurbitane-Type Triterpenoids from the Fruits ofMomordica charantiaand Their Cancer Chemopreventive Effects”. Journal of Natural Products 70 (8): 1233–1239. doi:10.1021/np068075p. ISSN 0163-3864.
- ^ Da-Cheng Wang, Hong-Yu Pan, Xu-Ming Deng, Hua Xiang, Hui-Yuan Gao, Hui Cai, and Li-Jun Wu (2007), "Cucurbitane and hexanorcucurbitane glycosides from the fruits of Cucurbita pepo cv dayangua". Journal of Asian Natural Products Research, volume 9, issue 6, pages 525–529.
- ^ a b c Chen, Jian-Chao; Zhang, Gao-Hong; Zhang, Zhong-Quan; Qiu, Ming-Hua; Zheng, Yong-Tang; Yang, Liu-Meng; Yu, Kai-Bei (2008). “Octanorcucurbitane and Cucurbitane Triterpenoids from the Tubers ofHemsleya endecaphyllawith HIV-1 Inhibitory Activity”. Journal of Natural Products 71 (1): 153–155. doi:10.1021/np0704396. ISSN 0163-3864.
- ^ Jian-Wen Tan, Ze-Jun Dong, Zhi-Hui Ding and Ji-Kai Liu (2002), "Lepidolide, a Novel Seco-ring-A Cucurbitane Triterpenoid from Russula lepida (Basidiomycetes)". Zeitschrift für Naturforschung Series C, volume 57C issue 11/12, pages 963-965.
- ^ Chen, Jian-Chao; Liu, Wu-Qing; Lu, Lu; Qiu, Ming-Hua; Zheng, Yong-Tang; Yang, Liu-Meng; Zhang, Xian-Min; Zhou, Lin et al. (2009). “Kuguacins F–S, cucurbitane triterpenoids from Momordica charantia”. Phytochemistry 70 (1): 133–140. doi:10.1016/j.phytochem.2008.10.011. ISSN 00319422.
- ^ J. C. Chen, R. R. Tian, M. H. Qiu, L. Lu, Y. T. Zheng, Z. Q. Zhang (2008), "Trinorcucurbitane and cucurbitane triterpenoids from the roots of Momordica charantia." Phytochemistry, volume 69, pages 1043–1048
- ^ Chen, Jian-Chao; Lu, Lu; Zhang, Xian-Ming; Zhou, Lin; Li, Zhong-Rong; Qiu, Ming-Hua (2008). “Eight New Cucurbitane Glycosides, Kuguaglycosides A – H, from the Root ofMomordica charantia L.”. Helvetica Chimica Acta 91 (5): 920–929. doi:10.1002/hlca.200890097. ISSN 0018019X.
- ^ Midori Takasaki, Takao Konoshima, Yuji Murata, Masaki Sugiura, Hoyoku Nishino, Harukuni Tokuda, Kazuhiro Matsumoto, Ryoji Kasai, and Kazuo Yamasaki (2003) "Anticarcinogenic activity of natural sweeteners, cucurbitane glycosides, from Momordica grosvenori". Cancer Letters, volume 198, pages 37–42
- ^ a b Sabira Begum, Mansour Ahmed, Bina S. Siddiqui, Abdullah Khan, Zafar S. Saify, and Mohammed Arif (1997), "Triterpenes, A Sterol and a Monocyclic Alcohol From Momordica Charantia." Phytochemistry, volume 44, issue 7, pages 1313-1320
- ^ Majekodunmi Fatope, Yoshio Takeda, Hiroyasu Yamashita, Hikaru Okabe, and Tatsuo Yamauchi (1990), "New cucurbitane trirterpenoids from Momordica charantia." Journal of Natural Products, volume 53, issue 6, pages 1491-1497.
- ^ Liu, Jie-Qing; Chen, Jian-Chao; Wang, Cui-Fang; Qiu, Ming-Hua (2009). “New Cucurbitane Triterpenoids and Steroidal Glycoside from Momordica charantia”. Molecules 14 (12): 4804–4813. doi:10.3390/molecules14124804. ISSN 1420-3049.
- ^ Harinantenaina, Liva; Tanaka, Michi; Takaoka, Shigeru; Oda, Munehiro; Mogami, Orie; Uchida, Masayuki; Asakawa, Yoshinori (2006). “Momordica charantia Constituents and Antidiabetic Screening of the Isolated Major Compounds”. CHEMICAL & PHARMACEUTICAL BULLETIN 54 (7): 1017–1021. doi:10.1248/cpb.54.1017. ISSN 0009-2363.
- ^ Liu, Jie-Qing; Chen, Jian-Chao; Wang, Cui-Fang; Qiu, Ming-Hua (2010). “One new cucurbitane triterpenoid from the fruits of Momordica charantia”. European Journal of Chemistry 1 (4): 294–296. doi:10.5155/eurjchem.1.4.294-296.131. ISSN 2153-2257.
- ^ Si Jian-yong, Chen Di-hua, Chang Qi and Shen Lian-gang (1996), Isolation and Determination of Cucurbitane-Glycosides from Fresh Fruits of Siraitia Grosvenorii. Journal of Integrative Plant Biology, volume 38, issue 6, pages, 489–494